|
With pyridine; In 1,4-dioxane; cyclohexane; at 25 - 32℃; for 2.5h;Product distribution / selectivity; |
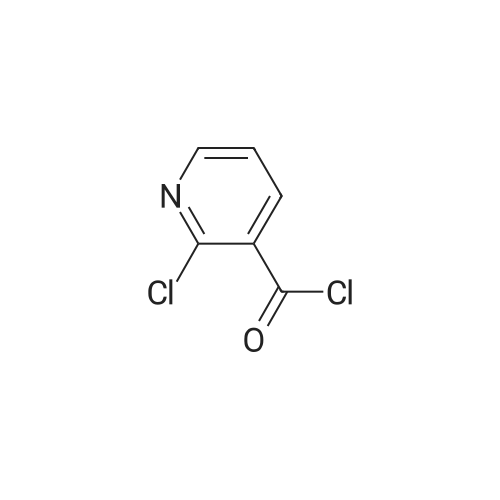
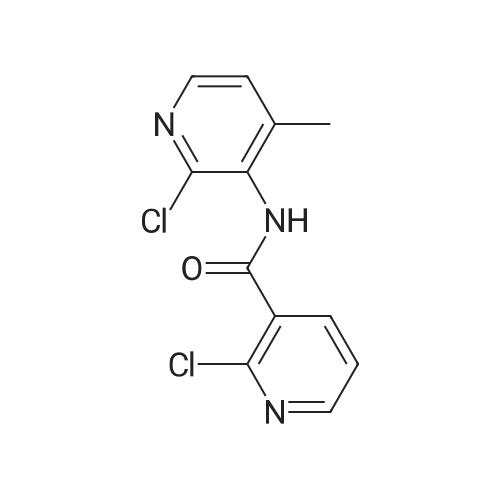
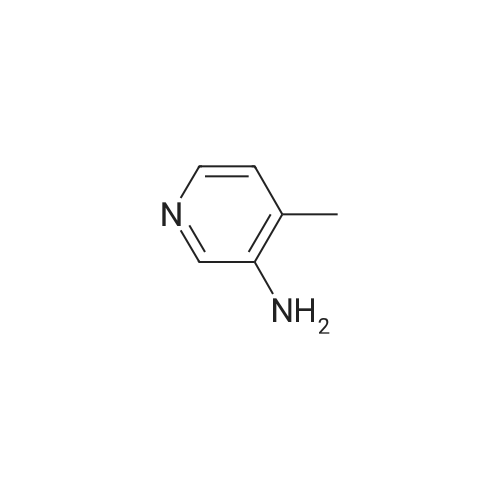
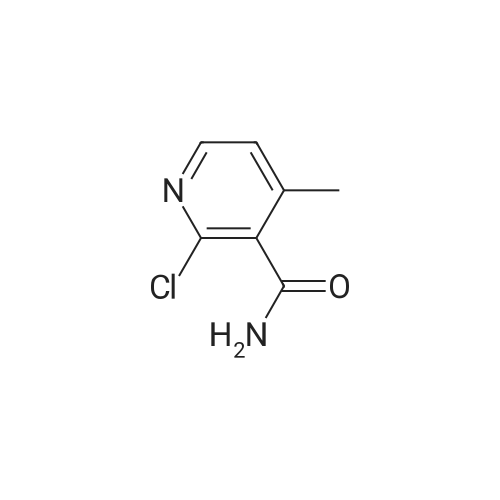
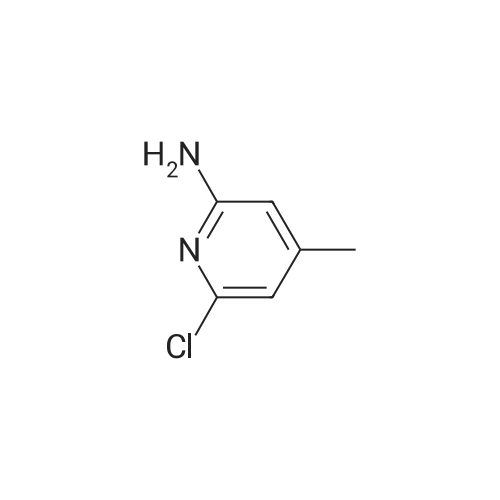
Example 6: Preparation of 2-Chloro-N-(2-chloro-4-methyl-3-pyridinyl)-3-pyridine carboxamide (V) [utilizing toluene as solvent during acylation and pyridine as organic base]2-Chloro nicotinic acid (41.5gms; 0.263moles) was added to toluene (125ml) and thionyl chloride (19ml; 0.26moles) and dimethyl formamide (2.5ml; 0.005moles) were added and the mixture refluxed at 800C -900C till completion of reaction. The reaction mixture was concentrated under reduced pressure and the residue diluted with dioxane (55ml). Pyridine (62.5ml; 0.77moles) was added to a mixture of 3-Amino-2-chloro-4-methyl pyridine of formula (111) stirred in cyclohexane (62.5ml). The acid chloride (IV) mixture in dioxane, EPO <DP n="21"/>was added to the mixture containing (III) and stirred for 2.5 hours at 25C to 32C. TLC monitoring of the reaction mixture showed lot of impurities. The solid separating out was filtered washed with cyclohexane. Compound (V) was dissolved in acetone (300ml), refiuxed, and concentrated to 50ml. The mixture was cooled between 5 and 100C, filtered and dried.Yield: 32gms % Yield: 64.9%. |
|
With N,N-dimethyl-aniline; In toluene; at 25 - 40℃; for 2.5h;Product distribution / selectivity; |
Example 11: Preparation of 2-Chloro-N-(2-chloro-4-methyl-3-pyridinyl)-3-pyridine carboxamide (V) [utilizing toluene as solvent and N,N-dimethyl aniline as organic base during acylation]2-Chloro nicotinic acid (165.75gms; 1.057moles) was added to toluene (542gms) and thionyl chloride (56.40gms; 0.477moles) and dimethyl formamide (5.9gms; 0.080moles) were added and the mixture refluxed at 80C-90C till completion of reaction. The reaction mixture was concentrated under reduced pressure and the residue diluted with toluene (542gms). 3-Amino-2-chloro-4-methyl pyridine (125gms; 0.877moles) of formula (III) was added to the mixture containing compound of formula (IV) at ambient temperature followed by addition of N,N-dimethyl aniline (127.12gms; 1.05moles). The reaction mixture was stirred for 2.5 hours at 250C to 4O0C. After completion of reaction, the reaction mixture was cooled to ambient temperature and neutralized with 10% sodium carbonate solution to pH 7.0 to 7.5. The solid separating out was filtered, washed with toluene and dried. Yield: 220-230 gms % Yield: 89-93%. Purity: 99% |
|
With pyridine; In 1,4-dioxane; cyclohexane; at 20℃; for 48h;Product distribution / selectivity; |
2-Chloro-N-(2-chloro-4-methyl-pyridin-3-yl)-nicotinamide: The procedure is carried out as described in Hargrave J. Med. Chem. 1991, 34, 2231-2241, which is hereby incorporated by reference in its entirety. To a solution of <strong>[133627-45-9]3-amino-2-chloro-4-methylpyridine</strong> (18.2 mmol) in 6:1 cyclohexane-dioxane (6 mL), and pyridine (5.75 mL) is added a solution of 2-chloronicotinoyl chloride (12.8 mmol) in 1,4-dioxane (5 mL). The resulting mixture is stirred at ambient temperature for 48 hours and the precipitate is filtered and washed with water. The solid is taken up in ethanol (17.5 mL) and aqueous NaOH (0.1 N, 3.6 mL). The solution is then heated to reflux for 2 hours, cooled to ambient temperature and stirred overnight. The solvent is removed under vacuum and water (10 mL) is added to residue, with stirring. The mixture is cooled to 1OC and the crystalline product is filtered, washed with cold water and dried under vacuum to give the desired product, 2-chloro-N-(2-chloro-4-methyl-pyridin-3-yl)-nicotinamide. |
|
With pyridine; In acetonitrile; at 20 - 45℃;Product distribution / selectivity; |
Step 3[00287] 2-Chloro-N-(2-chloro-4-methyl-pyridin-3-ylVnicotinamide: Pyridine (125 g, 1.58 mol, 1.10 equiv) was added to a solution of 2-chloro-4-methyl-pyridin-3-ylamine (204.6 g, 1.44 mol, 1.00 equiv) in acetonitrile (1500 ml) in a 2 liter 3 -necked round-bottom flask. 2- chloronicotinoyl chloride (270 g, 1.54 mol, 1.07 equiv) was added dropwise to the solution while maintaining the temperature at 20 C. The solution was allowed to react overnight while maintaining the temperature at 45 C in an oil bath. The solution was then diluted with water (2 L) and sodium carbonate was added till the pH of the solution reached 8. The solution was filtered, the filter cake was washed with water (100mLx3), and the filter cake was dissolved in tetrahydrofuran (3 L). The solution was decolorized by the addition of active carbon, and then filtered. The filtrate was then dried over sodium sulfate, concentrated in vacuo using a rotary evaporator. The product of 2-chloro-lambda/-(2-chloro-4-methyl-pyridin-3-yl) nicotinamide (32Og, purity: 94%, yield:79%) was obtained as a light red solid. The material was used in next step without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping