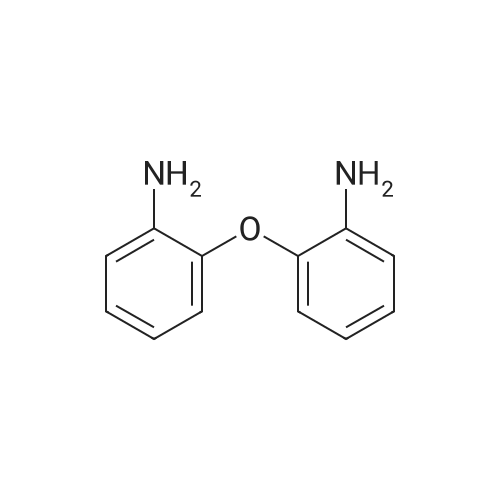
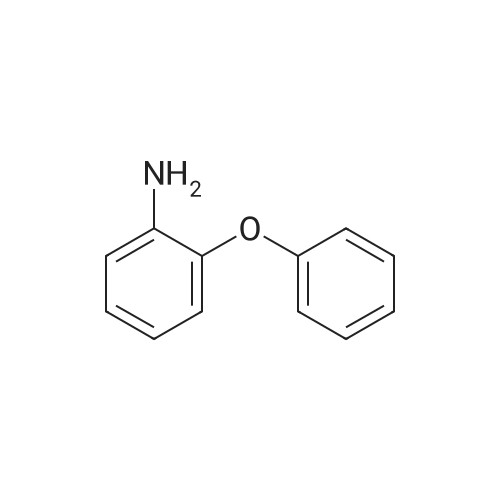
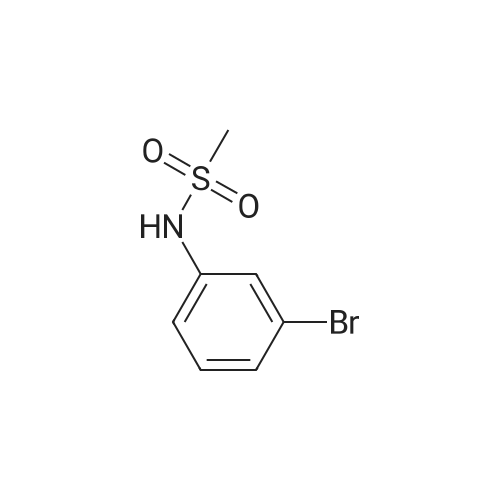
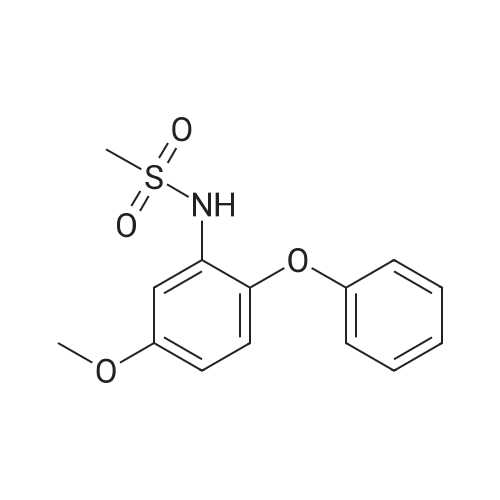
| 91.9% |
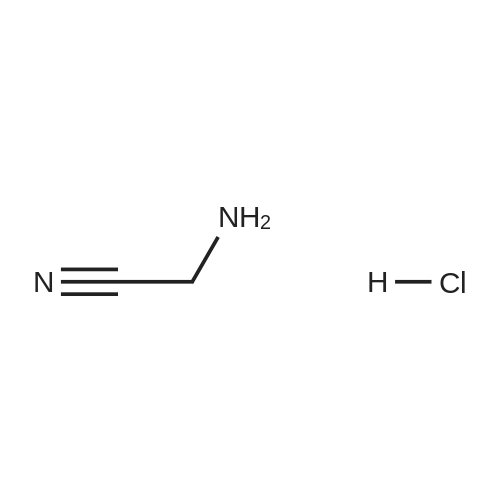
With hydrogenchloride; In pyridine; water; ethyl acetate; |
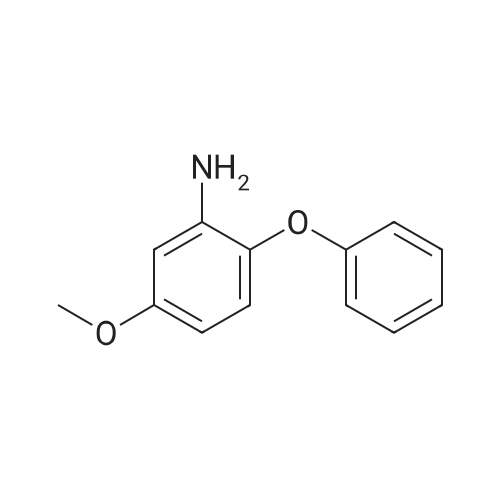
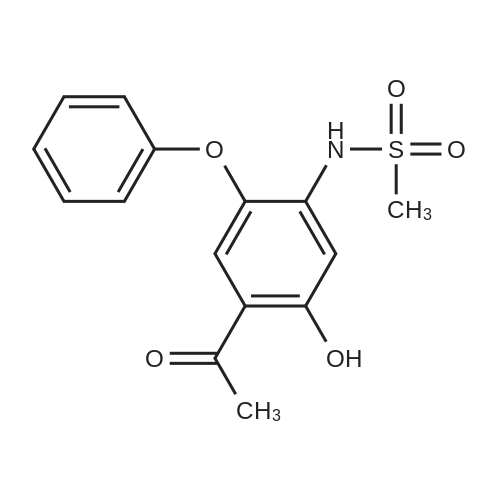
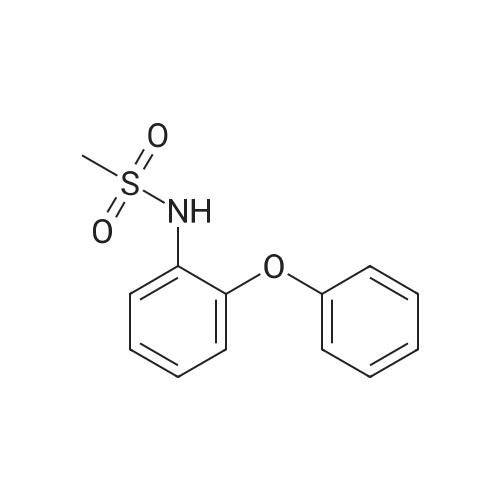
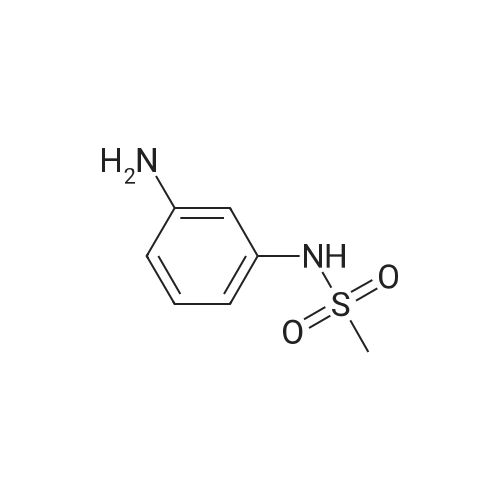
(4) 10.0 g of 3-amino-4-phenoxyanisole was dissolved in 50 ml of pyridine. Thereto was dropwise added 5.59 g of methanesulfonyl chloride in 10 minutes with ice-cooling. The mixture was stirred for 1 hour at 20-25 C. The reaction mixture was introduced into a mixture of 200 ml of ethyl acetate and 100 ml of water. The resulting organic layer was separated and washed with three 100-ml portions of 2N hydrochloric acid and then with a saturated aqueous sodium chloride solution. The organic layer was separated, dried with anhydrous magnesium sulfate, and subjected to distillation under reduced pressure to remove the solvent. The resulting crystal was recrystallized from isopropyl alcohol to obtain 12.5 g (yield: 91.9%) of 3-methylsulfonylamino4-phenoxyanisole having a melting point of 109.5-111 C. IR (KBr) cm-1: 3250, 1610, 1585, 1480, 1320, 1220, 1150 NMR (CDCl3)delta: 2.94 (3H, s), 3.81 (3H, s), 6.36-7.43 (9H, m) |
| 276.47 g |
With pyridine; at 5 - 30℃; for 1.5h;Green chemistry; |
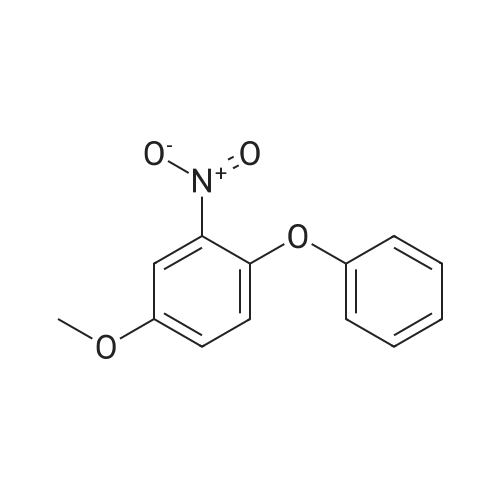
(1) 169 g of 4-methoxy-2 nitrophenol was dissolved in a mixture of 1500 ml of 70% ethanol and 40 ml of 4N HCl, the reaction liquid was heated to 75 C; Then, 196 g of iron powder was uniformly added to the reaction liquid in three batches in 20 minutes. The reaction was stirred rapidly and controlled to no starting material. After completion of the reaction, a saturated solution of sodium carbonate was added to adjust pH = 9 to obtain 2-amino-4-methoxyphenol. (2) 84 g of powdered potassium hydroxide prepared in (1) was added to 2-amino-4-methoxyphenol, stirred, and heated at 160 C for 3.5 hours, and the reaction was completed to obtain a dry salt; 1.89 g of copper powder and 188.4 g of bromobenzene were sequentially added thereto, and stirred at 180 C, mix to discoloration, heat to 200 C, and heat for 2 hours. After cooling, 1200 ml of water and 300 ml of diethyl ether were poured and distilled to give 3-amino-4-phenoxy-anisole. (3) The 3-amino-4-phenoxy-anisole prepared in (2) was dissolved in 800 ml of anhydrous pyridine, the solution was cooled to 5 C, and 137.5 g of methylsulfonyl chloride was added dropwise. After completion of the dropwise addition, the reaction was stirred at room temperature at 30 C, and a developing solvent (EA:PE volume ratio of 1:3) was added thereto, and the raw material was controlled until no raw material, and the reaction time was 1.5 h. After the reaction was completed, the reaction solution was poured into water and extracted with ethyl acetate. Twice, combining organic phases; Wash with 2N HCl until the aqueous layer is pH <7, then washed twice with water, dried over anhydrous magnesium sulfate, and then evaporated to ethyl ether. (5-methoxy-2-phenoxy - benzenesulfonamide quality 276.47g, a purity of 99.658%, 93.93% yield). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping