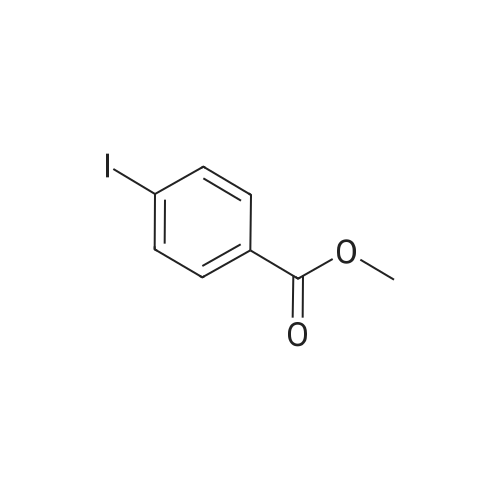
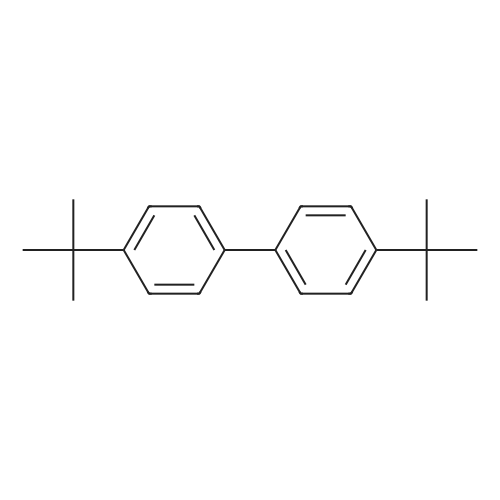
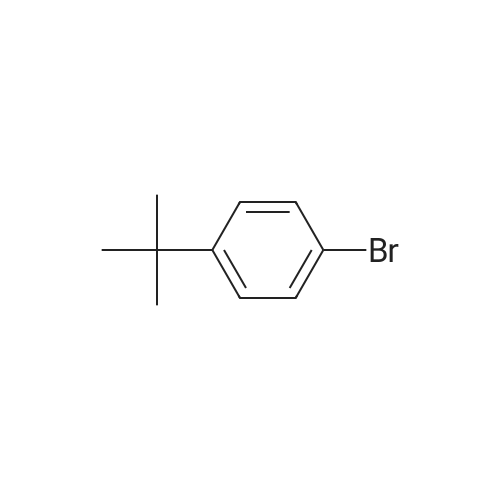
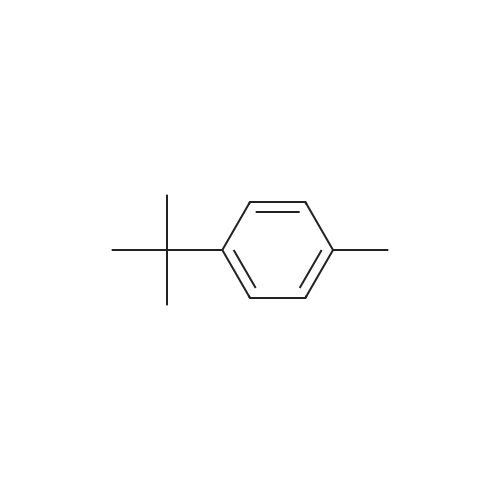
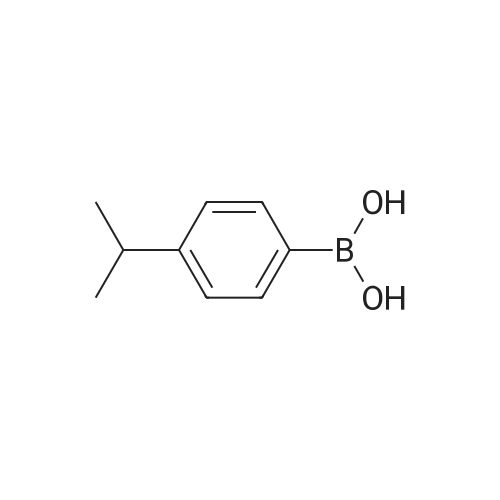
| 25% |
With oxygen; triethylamine;basolite C300; In dichloromethane; at 20℃; for 5h;Product distribution / selectivity; |
EXAMPLES[0008] MOF-199 (Basolite C300, Aldrich) , cyclohexylamine, triethylamine, phenylbornic acid, 4-nitrophenylboronic acid, 4-cyanophenylbornic acid, 4-chlorophenylboronic acid, 4-ter- burlyphenylboronic acid, 4- (dimehtylamino) -phenylboronic acid, naphthalene-1-bonronic acid and 1, 3, 5-trimethyoxybenzene were purchased form Aldrich Chemical Co.[0009] General homo-coupling reaction procedure:[0010] Cu3(BTC)2 (Basolite C300, provided by BASF,www.mof.basf.com, Aldrich catalogue number: 688614),cyclohexylamine, triethylamine, phenylboronic acid, 4- nitrophenylboronic acid, 4-cyanophenylboronic acid, 4- chlorophenylboronic acid, 4-tert-butylphenylboronic acid, 4-(dimethylamino) -phenylboronic acid, naphthyl-1-boronic acid and 1, 3, 5-trimethoxybenzene were purchased from AldrichChemical Co. Dichloromethane was purchased from FisherScientific International Inc. All starting materials were used without further purification. All experimental operations, unless otherwise noted, were performed in air. A mixture of arylboronic acid, (3.01 mmol) , cyclohexylamine (0.248 g, 286 muL, 2.51 mmol) and triethylamine (0.253 g, 348 muL, 2.49 mmol) were premixed and dissolved in 20 mL dichloromethane in a 50 mL round-bottom flask. MOF (0.100 g, 0.165 mmol, 0.495 mmol Cu(II)) or cupric acetate monohydrate (Cu (OAc) 2'H2O, 0.100 g, 0.501 mmol) was then added to the solution. The mixture was stirred at room temperature for 5 h, followed by filtration and washing with fresh dichloromethane. The excessdichloromethane in the filtrate was subsequently removed by rotovap . 1, 3, 5-trimethoxybenzene (0.168 g, 1.00 mmol) was added to the filtrate as internal standard for 1H NMR. Chemical shifts of all products in 1H NMR agree well with literature data .[0011] Biphenyl: 47percent yield of biphenyl (based onphenylboronic acid) was afforded when MOF was used; no biphenyl product was formed in the case of cupric acetate monohydrate. GC-MS, m/z+ 154.1; 4, 4' -dinitrobiphenyl : 87percent and 18percent yields of 4, 4' -dinitrobiphenyl (based on 4- nitrophenylboronic acid) were afforded when MOF and cupric acetate monohydrate were used, respectively. GC-MS, m/z+ 244.2; Biphenyl-4, 4' -dicarbonitrile : 92percent and 19percent yields of biphenyl-4, 4' -dicarbonitrile (based on 4-cyanophenylboronic acid) were afforded in the presence of MOF and cupric acetate monohydrate, respectively. 1.34 mmol (yield: 90percent) and 1.35 mmol (yield: 92percent) biphenyl-4, 4' -dicarbonitrile were observed in the second and third cycles, respectively. GC-MS, m/z+ 204.1; 4,4'-dichlorobiphenyl: 81percent and 8percent yields of 4,4'- dichlorobiphenyl (based on 4-chlorophenylboronic acid) were afforded in the presence of MOF and cupric acetatemonohydrate, respectively. GC-MS, m/z+ 223.1; 4, 4' -di-tert- butylbiphenyl : 25percent yield of 4, 4' -di-tert-butylbiphenyl (based on 4-tert-butylphenylboronic acid) was afforded when MOF was used, but none was formed in the case of cupric acetate monohydrate. GC-MS, m/z+ 266.1; N, N, N' ,N' -tetramethyldiphenyl- 4,4'-diamine: 43percent and 5percent yields of N, N, N', N'- tetramethyldiphenyl-4, 4' -diamine (based on 4- (dimethylamino) - phenylboronic acid) for MOF and cupric acetate monohydrate, respectively. GC-MS, m/z+ 240.1; 1, 1' -binaphthyl : 90percent and 6percent yields of 1, 1' -binaphthyl (based on naphthalyl-1-boronic acid) were afforded for MOF and cupric acetate monohydrate, respectively. GC-MS, m/z+ 254.2.[0012] Powder X-ray diffraction (PXRD) data were collected using a Bruker D8-Discover Psi-2Psi diffractometer in reflectance Bragg-Brentano geometry. Cu Ka radiation (lambda = 1.5406 A; 1,600 W, 40 kV, 40 mA) was focused using a planer Gobel Mirror riding the Ka line. A 0.6 mm divergence slit was used for all measurements. Diffracted radiation was detected using a Vantec line detector (Bruker AXS) (6° 2Psi sampling width) equipped with a Ni monochrometer . All samples were ground to ensure mono dispersity in the bulk, and then mounted onto a glass slide fixed on a sample holder by dropping powders and then leveling the sample surface with a wide-blade spatula. The best counting statistics were achieved by using a 0.02° 2Psi step scan from 1 - 50° with an exposure time of 0.4 s per step. The diffraction patterns collected for Cu3(BTC)2 both before and after homo-couplings are shown in Figures 4-10. Powder patterns of Cu3 (BTC)2 after three cycles alsoillustrated in Figure 11.[0013] FT-IR spectra of benzyltricarboxylic acid (BTC) and Cu3(BTC)2 (fresh and after coupling reaction) were obtained as KBr pellets using Nicolet 400 Impact spectrometer. FT-IR of recovered liquid after coupling reaction was performed on two clear KBr crystal plates. As shown in Figure 12 and 13, the C=O stretch of carboxylates in Cu3(BTC)2 absorbs at 1653 cm-1, whereas the C=O stretch of free carboxylic acid in BTC absorbs at 1734 cm'1, which is a strong characteristic peak for presence of any non-coordinated carboxylic groups. Recov... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping