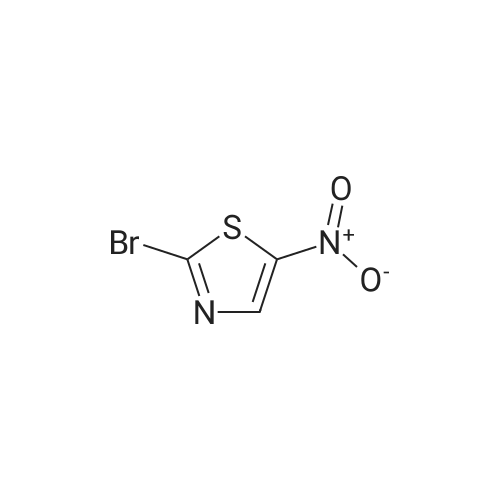
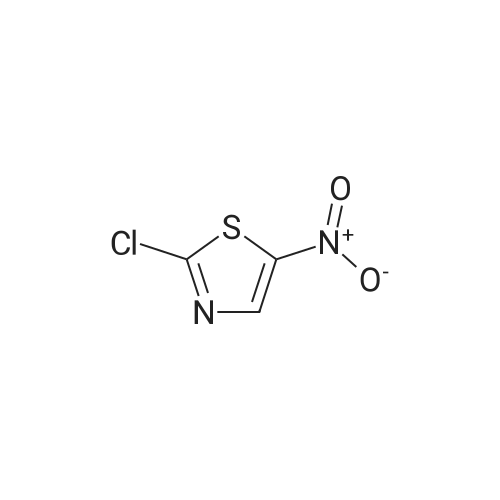
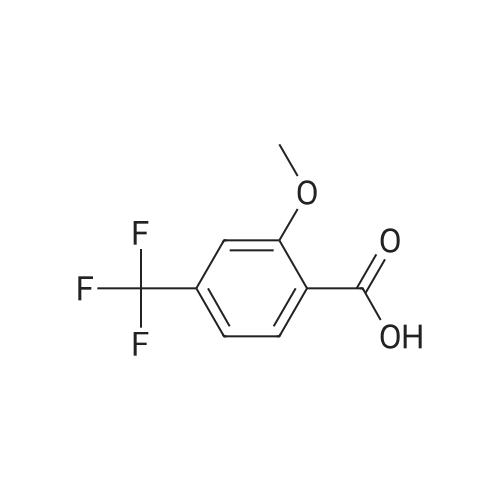
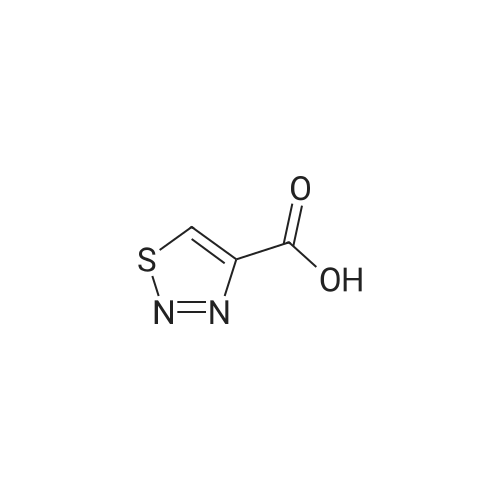
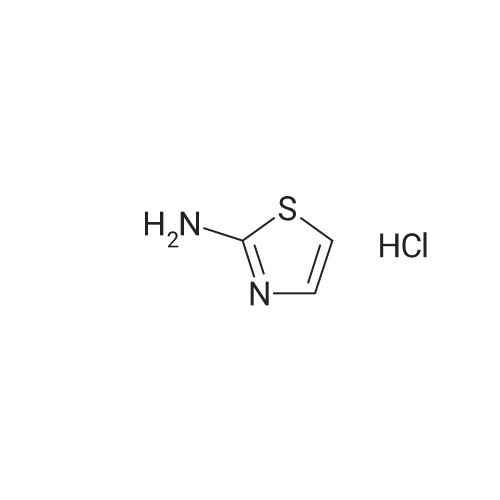
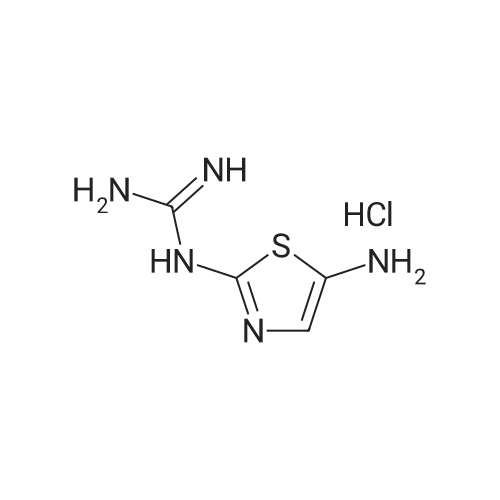
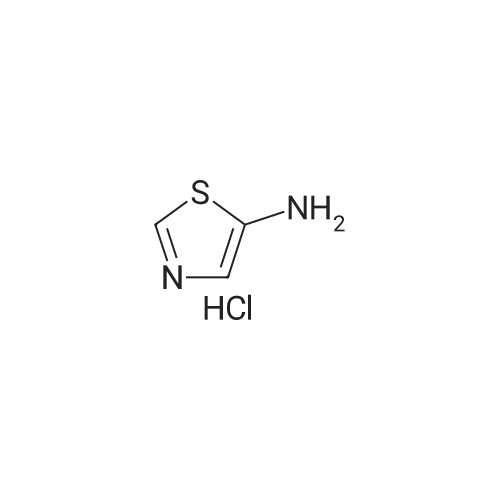
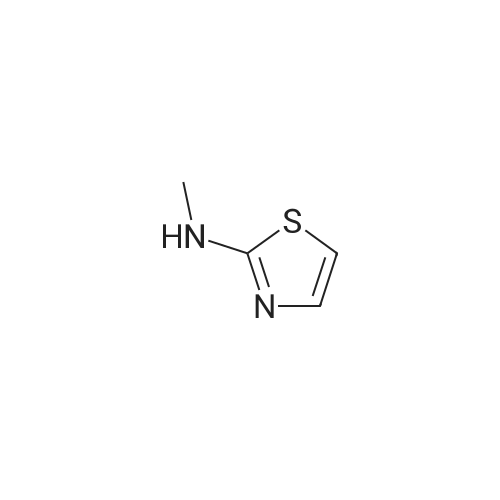
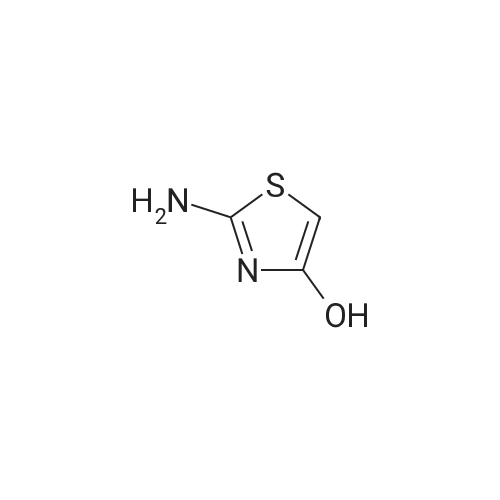
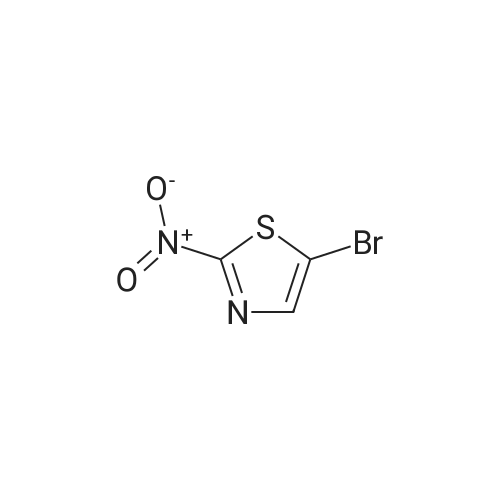
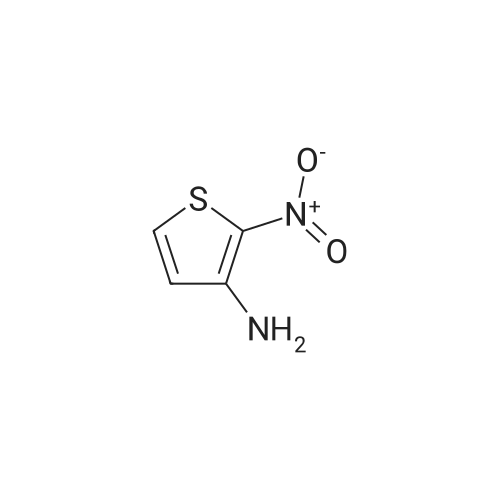
Synthesis and In Vitro Antibacterial Evaluation of Mannich Base Nitrothiazole Derivatives
Phelelisiwe S. Dube
;
Dylan Hart
;
Lesetja J. Legoabe
, et al.
Molbank,2024,2024(1):M1793.
DOI:
10.3390/M1793
More
Abstract: Nitrothiazole derivatives have been reported to exhibit activity against aerobic, anaerobic, and microaerophilic bacteria. This activity profile makes the nitrothiazole compound class an ideal lead source against Mycobacterium tuberculosis, which flourishes in varied environments with different oxygen concentrations. In this work, we investigated six nitrothiazole derivatives for antitubercular activity. The compounds exhibited potent activity, with compounds 9 and 10 possessing an equipotent MIC90 value of 0.24 μM. The compounds were investigated for cytotoxicity against HEK293 cells and hemolysis against red blood cells, and they demonstrated no cytotoxicity nor hemolytic effects, suggesting they possess inherent antitubercular activity.
Keywords:
nitrothiazole ;
Mannich bases ;
antitubercular activity ;
tuberculosis ;
Mycobacterium tuberculosis
Purchased from AmBeed:
121-66-4 ;
55981-09-4 ;
51322-75-9

Systematic analysis of gut bacterial carcinogen metabolism and its functional consequences
Boyao Zhang
;
George-Eugen Maftei
;
Bartosz Bartmanski
, et al.
bioRxiv,2024:2024.05.20.595058.
DOI:
10.1101/2024.05.20.595058
More
Abstract: Organic carcinogens, in particular DNA-reactive compounds, contribute to the irreversible initiation step of tumorigenesis through introduction of genomic instability. Although carcinogen bioactivation and detoxification by human enzymes has been extensively studied, carcinogen biotransformation by human-associated bacteria, the microbiota, has not yet been systematically investigated. We tested the biotransformation of 68 mutagenic carcinogens by 34 bacterial species representative for the upper and lower human gastrointestinal tract and found that the majority (41) of the tested carcinogens undergo bacterial biotransformation. To assess the functional consequences of microbial carcinogen metabolism, we developed a pipeline to couple gut bacterial carcinogen biotransformation assays with Ames mutagenicity testing and liver biotransformation experiments. This revealed a bidirectional crosstalk between gut microbiota and host carcinogen metabolism, which we validated in gnotobiotic mouse models. Overall, the systematic assessment of gut microbiota carcinogen biotransformation and its interplay with host metabolism highlights the gut microbiome as an important modulator of exposome-induced tumorigenesis.
Purchased from AmBeed:
446-86-6 ;
121-66-4 ;
607-35-2 ;
67-20-9 ;
59-87-0 ;
57-97-6 ;
5131-60-2 ;
512-56-1 ;
62-44-2 ;
6959-48-4 ;
84-65-1 ;
137-17-7 ;
117-39-5 ;
153-78-6 ;
1614-12-6 ;
298-81-7 ;
320-67-2 ;
99-55-8 ;
94-52-0 ;
101-61-1 ;
114-83-0 ;
64091-91-4 ;
53-96-3 ;
3817-11-6 ;
90-94-8 ;
613-13-8 ;
56-57-5 ;
91-64-5 ;
26148-68-5 ;
101-80-4 ;
139-65-1 ;
366-70-1 ;
389-08-2 ;
99-59-2 ;
132-32-1 ;
105650-23-5 ;
394-69-4 ;
117-39-5 ;
3544-23-8 ;
389-08-2 ;
320-67-2 ;
404-86-4 ;
82-28-0 ;
2832-40-8 ;
2475-45-8 ;
129-15-7 ;
103-33-3
...More



 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping