| 73 - 76% |
|
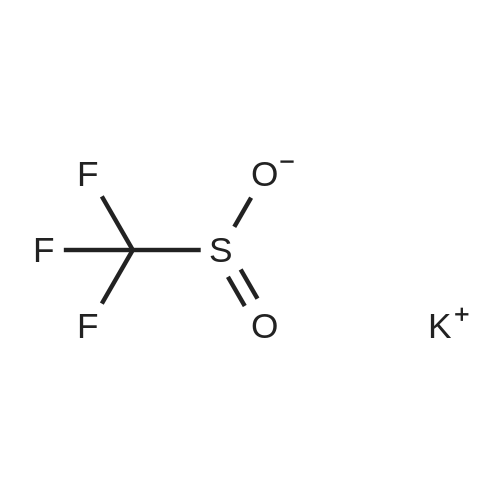
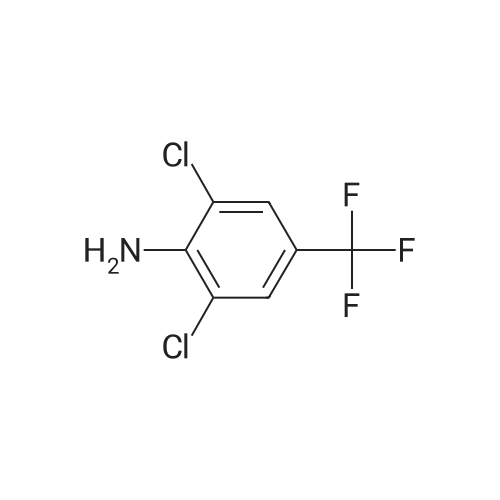
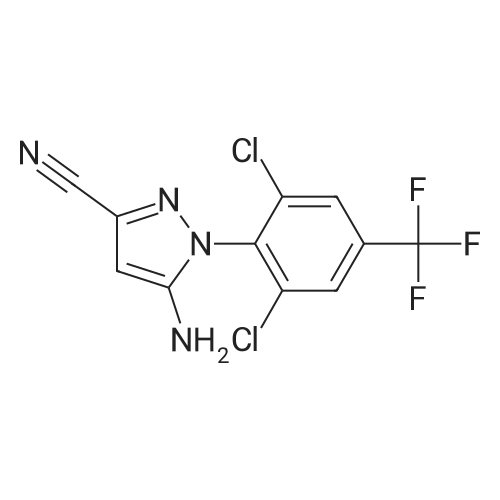
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Example 12; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3- carbonitrile with trimethylamine tosylate, sodium trifluoromethylsulfinate and thionyl- chloride, in 6.5 molar equivalents of tolueneThe preparation procedure was conducted as described above for example 1. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (76 % yield, 96 % purity by quantitative HPLC).; Example 14; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with trimethylamine tosylate, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneThe preparation procedure was conducted as described above for example 11. The crude product was crystallized from refluxing toluene (100 g) affording the title com- pound as a white crystalline powder (73 % yield, 98 % purity by quantitative HPLC). |
| 72 - 75% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Example 7; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with pyridine tosylate, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneThe preparation procedure was conducted as described above for example 1. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (75 % yield, 94 % purity by quantitative HPLC).; Example 11; Sulfinylation of 5-amino-1 -[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with thionylchloride, triethylamine hydrochloride and dosage of potassium trifluoromethylsulfinateWithin a 750 mL reactor with a mechanical stirrer and a thermometer were placed vac- uum dried triethylamine hydrochloride (51.1 g, 368 mmol), 147 g anhydrous toluene(6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile), and thionylchloride (35.7 g, 294 mmol) under an argon atmosphere. After cooling to 00C to 5 0C with external cooling, vacuum dried potassium trifluoromethylsulfinate (50.4 g, 296 mmol) was added in three equal portions every 10 min while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (79.5 g, 245 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was kept at 5 0C for 60 min and then heated to 35 0C within 45 min. The temperature of 35 0C was kept for another 10 hours before quenching the reaction with 200 g of sodium hydroxide solution (10 wt.%).The resulting suspension was diluted with 176 mL of ethylacetate. After phase separation the organic layer was washed once with sodium hydroxide solution (10 wt.%). After phase separation, the organic layer was analyzed by quantitative HPLC (79 % yield). The content of compound F was below 2.9 weight percent in the crude mixture (without solvent). The product was crystallized from a mixture of ethylacetate and toluene affording the title compound as a white crystalline powder (77.1 g, 75 % yield, 98 % purity by quantitative HPLC).; Example 16; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3- carbonitrile with pyridine tosylate, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of toluene EPO <DP n="30"/>The preparation procedure was conducted as described above for example 11. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (72 % yield, 98 % purity by quantitative HPLC). |
| 71% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Example 8; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with trimethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneThe preparation procedure was conducted as described above for example 1. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (71 % yield, 97 % purity by quantitative HPLC). |
| 67% |
With potassium fluoride; thionyl chloride;triethylamine hydrochloride; In toluene; at 0 - 50℃; for 5.08333h;Product distribution / selectivity; |
Example 5; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride under addition of potassium fluorideAn oven dried 100 mL 3 neck round bottom flask equipped with a magnetic stir bar, thermocouple, condenser, ISb inlet, and rubber septum, was charged with vacuum dried potassium fluoride (1.53 g, 26.1 mmol), anhydrous toluene (20.1 g), vacuum dried sodium trifluoromethylsulfinate (4.53 g, 29.0 mmol), and thionylchloride (3.76 g, 31.6 mmol) under nitrogen. The solution was cooled to 00C and triethylamine hydrochloride (5.44g, 39.5 mmol) was added slowly, controlling the temperature to less than 10 0C. Vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.48 g, 26.1 mmol, 99 % pure) was added at 00C. The solution was then quickly warmed to 50 0C, in less then 5 minutes, using a hot water bath. The reaction was allowed to stir for 5 hrs at 50 0C before quenching with 50 ml of saturated aqueous NaHCtheta3. The resulting suspension was diluted with 30 mL of ethylacetate and then allowed to phase separate. The aqueous phase was extracted with 30 mL ethyl acetate and the combined organic phases were washed with 30 ml of saturated aqueous NaCU3. The organic phase was concentrated under reduced pressure until dryness, affording the crude title compound (11.6 g, 67 % yield, 68.5 % purity). 1H-NMR (Bruker DRX-500, d6-DMSO): delta [ppm]: 8.33 (s), 7.57 (s). |
| 67% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Example 9; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with pyridine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneThe preparation procedure was conducted as described above for example 1. The crude product was crystallized from refluxing toluene (100 g) affording the title com- pound as a white crystalline powder (67 % yield, 95 % purity by quantitative HPLC).; Example 13; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3- carbonitrile with pyridine hydrochloride, sodium trifluoromethylsulfinate and thionylchlo- ride, in 6.5 molar equivalents of tolueneThe preparation procedure was conducted as described above for example 1. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (67 % yield, 95 % purity by quantitative HPLC). |
| 65% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Comparative Examples; In order to demonstrate the advantages of the inventive process, the following examples are conducted employing the preparation procedure given above for example 1. |
| 63 - 75% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Example 3; Sulfinylation of 5-amino-1 -[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile via addition of a mixture of sodium trifluoromethylsulfinate, triethylamine hydrochloride and thionylchloride to 5-amino-1-[2,6-dichloro-4- (trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrileWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 10 g anhydrous toluene under an argon atmosphere. After cooling to 0 - 5 0C with an ice bath thionylchloride (3.57 g, 30 mmol) was added while keeping the reaction temperature below 5 0C. After stirring for another 30 min the cooled sulfinic acid solution was added at once to a stirred suspension of vacuum dried 5-amino-1-(2,6-dichloro-4- trifluoromethyl-phenyl)-1 H-pyrazole-3-carbonitrile (8.03 g, 25 mmol, 99 % purity) in 5 g toluene with a temperature of 50 0C. The temperature of 50 0C was kept for another 5 hours before quenching the reaction with 50 ml. of saturated NaHCU3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separation the organic layer was washed once with saturated NaHCU3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (7.25 g, 63 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Example 11; Sulfinylation of 5-amino-1 -[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with thionylchloride, triethylamine hydrochloride and dosage of potassium trifluoromethylsulfinateWithin a 750 mL reactor with a mechanical stirrer and a thermometer were placed vac- uum dried triethylamine hydrochloride (51.1 g, 368 mmol), 147 g anhydrous toluene(6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile), and thionylchloride (35.7 g, 294 mmol) under an argon atmosphere. After cooling to 00C to 5 0C with external cooling, vacuum dried potassium trifluoromethylsulfinate (50.4 g, 296 mmol) was added in three equal portions every 10 min while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (79.5 g, 245 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was kept at 5 0C for 60 min and then heated to 35 0C within 45 min. The temperature of 35 0C was kept for another 10 hours before quenching the reaction with 200 g of sodium hydroxide solution (10 wt.%).The resulting suspension was diluted with 176 mL of ethylacetate. After phase separation the organic layer was washed once with sodium hydroxide solution (10 wt.%). After phase separation, the organic layer was analyzed by quantitative HPLC (79 % yield). The content of compound F was below 2.9 weight percent in the crude mixture (without solvent). The product was crystallized from a mixture of ethylacetate and toluene affording the title compound as a white crystalline powder (77.1 g, 75 % yield, 98 % purity by quantitative HPLC).; Example 15; Sulfinylation of 5-amino-1-[2,6... |
| 57% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Comparative Examples; In order to demonstrate the advantages of the inventive process, the following examples are conducted employing the preparation procedure given above for example 1. |
| 55% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Comparative Examples; In order to demonstrate the advantages of the inventive process, the following examples are conducted employing the preparation procedure given above for example 1. |
| 44% |
|
Example 1; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and thionylchloride, in 6.5 molar equivalents of tolueneWithin a 3-neck, 50 ml. round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed vacuum dried sodium trifluoromethylsulfinate (4.29 g, 27.5 mmol), vacuum dried triethylamine hydrochloride (5.16 g, 37.5 mmol), and 13 ml. an- hydrous toluene (6.5 molar equivalents relative to 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under an argon atmosphere. After cooling to 00C to 5 0C with an ice bath, thionylchloride (3.57 g, 30 mmol) was added slowly while keeping the reaction temperature below 5 0C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-1 H-pyrazole-3- carbonitrile (8.03 g, 25 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 50 ml. of saturated NaHCO3 solution. The resulting suspension was diluted with 30 ml. of ethylacetate. After phase separa- tion the organic layer was washed once with saturated NaHCtheta3 solution and concentrated under reduced pressure until dryness. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (8.06 g, 70 % yield, 94 % purity by quantitative HPLC). 1H-NMR (Bruker DRX-500, d6- DMSO): delta [ppm]: 8.33 (s), 7.57 (s).; Example 17; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H-pyrazole-3- carbonitrile with triethylamine tosylate, sodium trifluoromethylsulfinate and thionylchlo- ride, in 6.5 molar equivalents of tolueneThe preparation procedure was conducted as described above for example 1. The crude product was crystallized from refluxing toluene (100 g) affording the title compound as a white crystalline powder (44 % yield, 76 % purity by quantitative HPLC). No formation of insoluble material (as with diethylamine tosylate as the amine acid complex, compare example C4 of Table 3) was observed. |
| 6 - ~ 8% |
With phosphorus tribromide;dimethylamine p-toluenesulfonate; at -15 - 57℃; for 2 - 14h;Product distribution / selectivity; |
Comparative Example 13; [0123] Direct sulfinylation of N-phenyl pyrazole starting material (III) according to known methods was tested. As such, sulfinylation was attempted using CF3SO2Na in the presence of a halogenating agent such as POCl3, SOCl2 or PBr3. <n="35"/>(III) (II)The reaction reagents and conditions tested are provided in Table I below. <n="36"/>Table IUl <n="37"/>The results are provided in Table II below: Table II[0124] The reaction proceeded to the desired product, Fipronil, when SOCl2 or POCl3 were used as halogenating agents. However, PBr3 did not yield the desired product, or at least not in acceptable yield (about 6%~8% (II) in the reaction mixture, according to HPLC). |
| 57.3%Chromat. |
|
Example 6; Sulfinylation of 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-1 H- pyrazole-3-carbonitrile with triethylamine hydrochloride, sodium trifluoromethylsulfinate and phosporoxychlorideWithin a 3-neck, 50 mL round bottom flask equipped with a magnetic stirrer bar and a thermometer were placed sodium trifluoromethylsulfinate (8.84 g, 55.0 mmol) and 40 mL dried toluene (6.8 molar equivalents relative to 5-amino-1-[2,6-dichloro-4- (trifluoromethyl)phenyl]-1 H-pyrazole-3-carbonitrile) under a nitrogen atmosphere. After cooling to 00C to 5 0C with an ice bath, phosphoroxychloride (9.20 g, 60.0 mmol) was added slowly while keeping the reaction temperature below 5 0C. After complete addi- tion of phosphoroxychloride, triethylamine hydrochloride (10.32g, 75.0 mmol) was added at 5C. After stirring for another 30 min, vacuum dried 5-amino-1-(2,6-dichloro-4- EPO <DP n="27"/>trifluoromethyl-phenyl)-1 H-pyrazole-3-carbonitnle (16.06 g, 50 mmol, 99 % purity) was added at 5 0C, and the reaction mixture was heated to 50 0C within 5 min by a preheated water bath. The temperature of 50 0C was kept for another 6 hours before quenching the reaction with 100 g of 10% NaHCO3 solution. The resulting suspension was diluted with 100 ml. of ethylacetate. After phase separation, the organic layer was analyzed by quantitative HPLC (57.3% yield). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping