|
With triphenylphosphine; diethylazodicarboxylate; In dichloromethane; at 25℃; for 2h; |
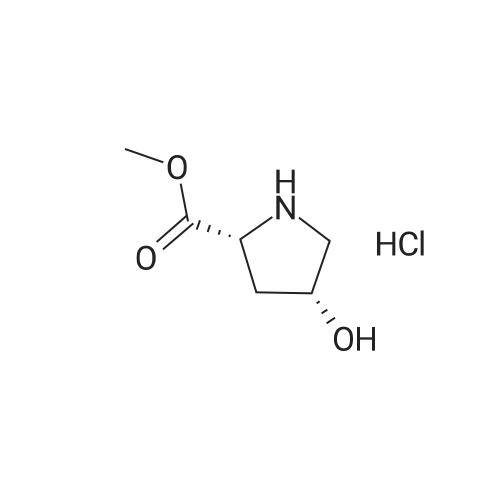
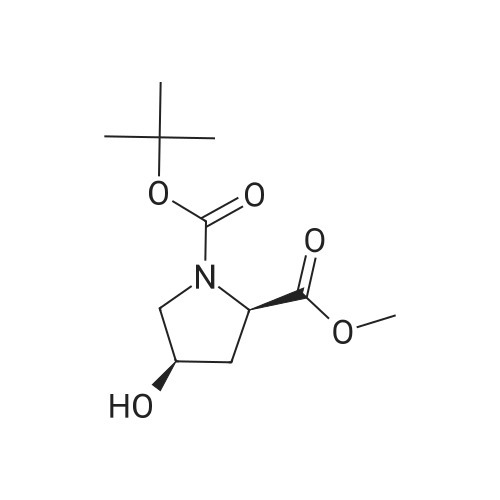
Example 1 (4S)-4- ({4-[(3-Chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl}oxy)-N- cyclopropyl-1-methyl-D-prolinamide Example 1 HATU (0.23g) was added to an agitated solution of (4Y)-4- ( {4- [ (3-CHLORO-2- fluorophenyl) AMINO]-7-METHOXYQUINAZOLIN-6-YL} OXY)-1-METHYL-D-PROLINE (7) (0.18g), cyclopropylamine (34.4mg) and DIPEA (156mg) IN METHYLENE CHLORIDE (5M1). After 16hrs the reaction mixture was reduced in vacuo. The residues were re-dissolved in methylene chloride and washed with sodium hydroxide solution (2M) and water. The organic phase was then purified by column chromatography on silica eluting with increasingly polar mixtures of METHANOL/METHYLENE CHLORIDE (0/100-10/90). THE fractions containing the desired product were combined and evaporated to a foam which was triturated with diethylether to give the title compound as a white solid. (0. 15G). LH NMR Spectrum : (DMSO d6) 0.40-0. 48 (m, 2H), 0.57-0. 64 (M, 2H), 2.05-2. 14 (M, 1H), 2.28 (s, 3H), 2.33-2. 45 (M, 1H), 2.48-2. 56 (M, 1H + DMSO), 2. 61-2. 70 (M, 1H), 3.08 (t, 1H), 3.64 (dd, 1H), 3.94 (s, 3H), 5.06 (M, 1H), 7.20 (s, 1H), 7.28 (t, 1H), 7.44-7. 56 (M, 2H), 7.65 (s, 1H), 7.87 (d, 1H), 8.35 (s, 1H), 9.63 (s, 1H); Mass SPECTRUM : (M+H) + 486. 44 The starting material 1, 2-PYRROLIDINEDICARBOXYLIC acid, 4-hydroxy-, 1- (1, 1-DIMETHYLETHYL) 2- methyl ester, (2R, 4R) (2) was prepared as follows: 1- [3- (DIMETHYLAMINO) PROPYL]-3-ETLIYLCARBODIIMIDE hydrochloride (14.73 g) was added to a stirred suspension of 1, 2-PYRROLIDINEDICARBOXYLIC acid, 4-hydroxy-, l- (l, l-dimethylethyl) ester, (2R, 4R) (1) (13.65 g), DIMETHYLAMINOPYRIDINE (21.65 g) and methanol (5.67 g) in methylene chloride (400 ml) and the reaction mixture was stirred at room temperature for 16 hours. The reaction mixture was washed with citric acid (1.0 M), saturated aqueous sodium bicarbonate solution and saturated brine, dried over MgSO4, filtered and evaporated. The residues were then purified by column chromatography on silica eluting with increasingly polar mixtures of METHANOL/METHYLENE chloride (1/99-5/95). The desired product fractions were combined and evaporated to give 1, 2-PYNOLIDINEDICARBOXYLIC acid, 4-hydroxy-, L- (L, L-DIMETHYLETHYL) 2- methyl ester, (2R, 4R) (2) as a white crystalline solid, (5.9 g). 1H NMR Spectrum : (DMSO d6) 1.32 + 1.38 (2s, 9H), 1.76-1. 87 (M, 1H), 2.24-2. 28 (M, 1H), 3.06-3. 15 (M, 1H), 3.42- 3. 51 (m, 1H), 3.60 + 3.63 (2s, 3H), 4.15-4. 24 (M, 2H), 4.92-5. 00 (M, 1H). Starting material 1, 2-Pyrrolidinedicarboxylic acid, 4-hydroxy-1- (1, 1-dimethylethyl) ester, (2R, 4R), (1), (Boc-D-cis-hyp-OH) is commercially available Starting material (3) was prepared as follows: 6-Acetoxy-4-chloro-7-methoxyquinazoline, (Example 25-5 of in W001/66099 ; 10. 0g, 39.6 MMOLE) was added in portions to a stirred 7N methanolic ammonia solution (220 ML) cooled to 10C in an ice/water bath. After stirring for one hour the precipitate was filtered, washed with diethylether and dried thoroughly under high vacuum to give 4-chloro-7- METHOXYQUINAZOLIN-6-OL (3) (5.65g, 67.8%) ; 1H NMR Spectrum : (DMSO d6) 3.96 (s, 3H); 7.25 (s, 1H); 7.31 (s, 1H); 8.68 (s, 1H) ; Mass Spectrum: (M+H) + 211. The starting material (4) was prepared as follows: Di-ethyl azodicarboxylate (5.71g) was added slowly to a stirred suspension of 1,2- PYNOLIDINEDICARBOXYLIC acid, 4-hydroxy-, L- (L, L-DIMETHYLETHYL) 2-methyl ester, (2R, 4R) (2) (5.9g), 4-CHLORO-7-METHOXYQUINAZOLIN-6-OL (3) (4.6g) AND TRIPHENYLPHOSPHINE (8.6g) in methylene chloride (400 ML) at 25C under an atmosphere of nitrogen and the reaction mixture was stirred for 2 hours. The reaction mixture was then evaporated to ? volume and purified by column chromatography on silica eluting with increasingly polar mixtures of METHANOL/METHYLENE chloride (1/99-3/97). The desired product fractions were combined and evaporated to give 1-tert-butyl 2-methyl (2R, 4S)-4-[(4-chloro-7-methoxyquinazolin-6- yl) oxy] pyrrolidine-1, 2-dicarboxylate (4) as a pale yellow gum. This was used in the preparation of (5) without further purification. The starting material methyl (4S)-4-({4-[(3-CHLORO-2-FLUOROPHENYL) AMINO]-7- METHOXYQUINAZOLIN-6-YL} OXY)-D-PROLINATE HYDROCHLORIDE (5) was prepared as follows: 4. 0M HCl in Dioxane (15 ml) was added to a suspension of 1-tert-butyl 2-methyl (2R, 4S)-4- [(4-chloro-7-methoxyquinazolin-6-yl0 oxy] pyrrolidine-1, 2-dicarboxylate (4) AND 3-CHLORO-2- fluoroaniline (2.89g) in acetonitrile (400 ML) and the reaction mixture was stirred and heated at 70C for 3 hours. The resulting precipitate was filtered hot and washed with acetonitrile and diethylether and dried under vacuum to give methyl (4S)-4- (14- [ (3-CLILORO-2- FLUOROPHENYL) AMINO]-7-METHOXYQUINAZOLIN-6-YL} OXY)-D-PROLINATE HYDROCHLORIDE (S) as an off- white solid, (6. 3G). TH NMR Spectrum : (DMSO D6) 2.46-2. 60 (M, 2H), 3.37-3. 46 (M, 1H), 3.71 (s, 3H), 3.89-3. 98 (m, 4H), 4.53 (t, 1H), 5.42 (M, 1H), 7.29 (t, 1H), 7.38-7. 48 (M, 2H), 7.55 (t, 1H), 8.64 (s, 1H), 8.75 (s, 1H)... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping