Evaluation of Non-Saccharomyces Yeast for Low-Alcohol Beer Production
Krystian Klimczak
;
Monika Cioch-Skoneczny
;
Aleksander Poreda
Appl. Sci.,2024,14(15):6755.
DOI:
10.3390/app14156755
More
Abstract: Among many methods to produce low/no-alcohol beers, using special yeasts has gained a substantial interest in the brewing industry. This approach relies on the fact that many non-Saccharomyces yeasts do not utilize maltose, which is the main sugar found in brewer’s wort. Additionally, these yeasts may allow the production of a beer with unique sensory characteristics. The aim of the study was to evaluate the potential of 18 non-Saccharomyces yeast strains in the production of low-alcohol beer. As a control strain, S. cerevisiae US-05 was used. The study consisted of two parts: microbiological evaluation and small-scale fermentations. In the microbiological part, ability to ferment sugars found in a wort, resistance to stress factors, phenolic off-flavor production, and enzymatic activities of β-glucosidase and β-lyase were evaluated. In the second part of the study, yeasts were used to produce a beer from 9.3 °Plato wort. During the fermentation, its dynamics was analyzed. The obtained beers were analyzed regarding their alcohol content, pH, acidity, and color. All of the evaluated strains produced low levels of alcohol. Two of the evaluated strains were characterized by especially high β-glucosidase activity. Based on the obtained results, six of the evaluated strains are promising in brewing.
Keywords:
beer ;
non-Saccharomyces ;
low-alcohol beer
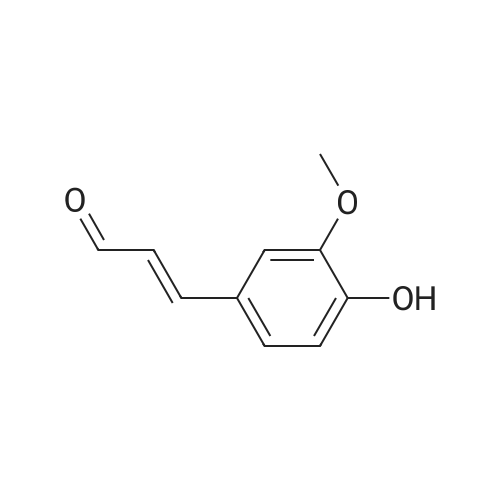
Purchased from AmBeed:
1135-24-6

Enhancing the anticancer effects of Apiaceae spice phytochemicals.
Scott, Jared Lee
;
University of Louisville,2024.
DOI:
/
More
Abstract: Breast cancer (BC) is the second greatest contributor to the death of women, second only to heart disease, and is the most common type of cancer. BC treatments involve the administration of adjuvant chemotherapies which often have side effects that prevent patients from completing the full course of drugs or the refusal to take these potentially lifesaving treatments. Many chemotherapy drugs are developed from plants, and some plant extracts can exhibit significant anticancer activities while also having less toxic side effects. However, these potential "plant therapeutics" suffer from poor oral bioavailability. The Apiaceae plant family consists of several species that are used as culinarily spices including anise, celery, cumin, and coriander, all of which have demonstrated antioxidant, chemopreventive, and anticancer activities. One method to improve the systemic distribution of anticancer phytochemicals is their encapsulation in naturally produced membrane bound nanoparticles known as exosomes. Exosomes are produced by most eukaryotic organisms, as well as some prokaryotes, and are involved in cell-to-cell communication through the delivery of proteins, nucleic acids, and small molecules from one cell to another. Exosomes are found in many extracellular fluids including blood, urine, and milk. Bovine milk exosomes represent a scalable source of exosomes that are already present in the human diet and have been explored as a drug delivery system that can increase effectiveness and improve bioavailability. To enhance the loading potential and anticancer bioactivity of Apiaceae phytochemicals, an acid hydrolysis (AH) of the glycoside compounds present in ethanolic spice extracts was performed on eight ethanolic spice extracts. The antiproliferative effects of AH extracts and exosomal formulations were assayed with three model types of BC cells. Cumin was characterized in greater detail as these extracts had the highest concentration of terpenoids and alkaloids while also having significant concentrations of phenolics and responded well to AH with increased antiproliferative activity and exosomal loading. Extracts and exosomal formulations exhibited broad antiproliferative effects with lower IC50s in the extracts delivered with exosomes. The phytochemical contents of AH-cumin extracts and exosomal formulations were assayed with HPLC-DAD, LC-MS/MS, and GC-MS, while the potential anticancer mechanisms of these treatments were investigated in triple negative BC (TNBC). AHcumin extracts were determined to have numerous phenolic compounds, many of which have known anticancer mechanisms, in addition to several alkaloids and lipid compounds, some of which have activities that could contribute to the anticancer effects observed. Mechanistically, AH-cumin extracts and exosomal formulations were shown to interact with multidrug resistance proteins and inhibit lipid metabolism in TNBC cells. These results indicate that acid hydrolyzed cumin extracts delivered through exosome nanoparticles represent a possible avenue towards the development of novel treatments for TNBC, the hardest type of BC to treat.
Purchased from AmBeed:
331-39-5 ;
621-82-9 ;
1135-24-6 ;
491-70-3 ;
327-97-9 ;
520-36-5 ;
501-98-4 ;
121-34-6 ;
117-39-5 ;
1617-53-4 ;
91-64-5 ;
530-57-4 ;
20283-92-5

New ferulic acid and amino acid derivatives with increased cosmeceutical and pharmaceutical potential
Janus, Ewa
;
Pinheiro, Luan Ramalho
;
Nowak, Anna
, et al.
Pharmaceutics,2022,15(1):117.
DOI:
10.3390/pharmaceutics15010117
PubMed ID:
36678746
More
Abstract: Ferulic acid (FA) has been widely used in the pharmaceutical and cosmetics industry due to its, inter alia, antioxidant, antiaging and anti-inflammatory effects This compound added to cosmetic preparations can protect skin because of its photoprotective activity. However, the usefulness of FA as a therapeutic agent is limited due to its low solubility and bioavailability. The paper presents the synthesis, identification, and physicochemical properties of new FA derivatives with propyl esters of three amino acids, glycine (GPr[FA]), L-leucine (LPr[FA]), and L-proline (PPr[FA]). The NMR and FTIR spectroscopy, DSC, and TG analysis were used as analytical methods. Moreover, water solubility of the new conjugates was compared with the parent acid. Both ferulic acid and its conjugates were introduced into hydrogel and emulsion, and the resulting formulations were evaluated for stability. Additionally, in vitro penetration of all studied compounds from both formulations and for comparative purposes using Franz diffusion cells was evaluated from the solution in 70% (v/v) ethanol. Finally, cytotoxicity against murine fibroblasts L929 was tested. All of the analyzed compounds permeated pig skin and accumulated in it. LPr[FA] and PPr[FA] were characterized by much better permeability compared to the parent ferulic acid. Additionally, it was shown that all the analyzed derivatives are characterized by high antioxidant activity and lack of cytotoxicity. Therefore, they can be considered as an interesting alternative to be applied in dermatologic and cosmetic preparations.
Keywords:
antioxidant activity ;
antiaging ;
ferulic acid ;
new ferulic acid and amino acid derivatives ;
skin permeation ;
toxicity ;
vehicles containing new ferulic acid and amino acid derivatives
Purchased from AmBeed:
1135-24-6


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping