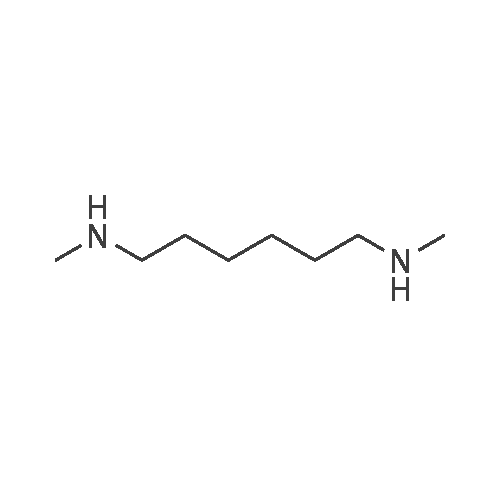
| 85 - 90% |
In N,N-dimethyl acetamide; at 90℃; for 12 - 16h; |
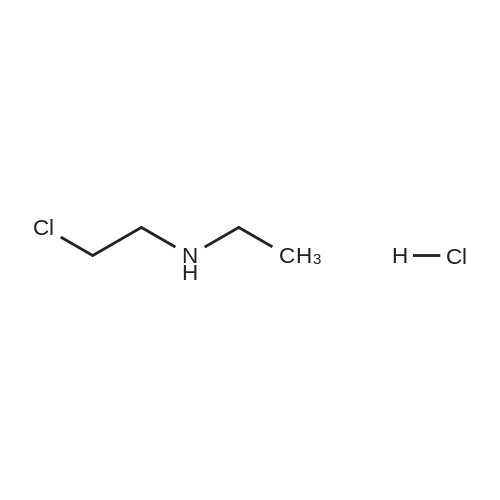
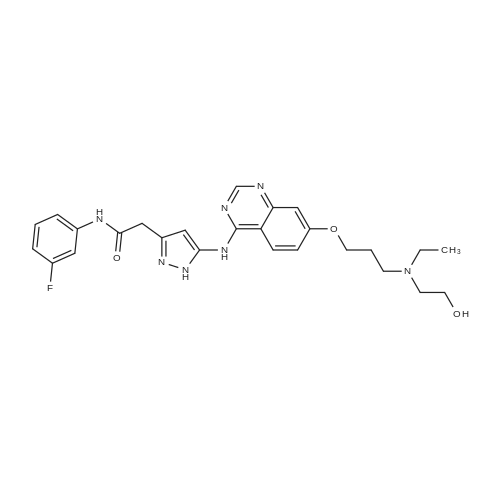
2-(3-[7-(3-Chloropropoxy)quinazolin-4-yl]amino}-1H-pyrazol-5-yl)-N-(3-fluorophenyl)acetamide and 2-(ethylamino)ethanol (ideally 12 molar equivalents) were added to N,N-dimethylacetamide under an inert atmosphere (such as provided by nitrogen) and the mixture heated to 90° C. with stirring. After a period of 12-16 hours (ideally 12 hours) the reaction is cooled back to about 85° C. and water added in a controlled manner to maintain the temperature between 80-85° C. The batch is adjusted to 80° C. and seeded with crystals of the preferred form of the product (ideally an amount of about 1percent of the expected yield). The mixture was cooled to 20° C. in a carefully controlled manner over a period of about 20 hours so as to crystallise the product in the required form and of a size sufficient to afford a good filtration rate. The product is then filtered and washed with a mixture of water/N,N-dimethylacetamide and acetonitrile and suitably deliquored to afford a hydrated form of the product. Following this, the cake is slurried in situ for a period (ideally 2 hours) with warm acetonitrile (ideally at a temperature of 40° C.) then filtered, washed with more acetonitrile and then dried (in vacuo or under a stream of nitrogen) to afford the almost anhydrous 2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}-quinazolin-4-yl)amino]-1H-pyrazol-5-yl}-N-(3-fluorophenyl)acetamide as an off-white solid in a yield of 85-90percent. 1H-NMR (DMSO d6): 10.55 (s, 1H), 9.45 (br s, 1H), 8.98 (s, 1H), 8.8 (d, 1H), 7.63 (pr of m, 1H), 7.47 (pr of d, 1H), 7.37 (m, 2H), 7.32 (d, 1H), 6.9 (m, 1H), 6.77 (s, 1H), 4.32 (t, 2H), 3.83 (br s, 2H), 3.76 (t, 2H), 3.35 (m, 2H), 3.25 (m, 4H), 2.25 (m, 2H), 1.25 (t, 3H): MS (+ve ESI): 508.4 (M+H)+. |
| 85 - 90% |
In ISOPROPYLAMIDE; at 80 - 90℃; for 12 - 16h;Product distribution / selectivity; |
2-(3-[7-(3-Chloropropoxy)quinazolin-4-yl]amino}-lH-pyrazol-5-yl)-N-(3- fluorophenyl)acetamide and 2-(ethylamino)ethanol (12 molar equivalents) were added to N,N-dimethylacetamide under an inert atmosphere (such as provided by nitrogen) and the mixture heated to 9O0C with stirring. After 12 hours, water was added in a controlled manner and the batch seeded with product whilst hot. The mixture was cooled to 2O0C in a carefully controlled manner to crystallise the product in the required form. The product was then filtered and washed with a mixture of water/ N,N-dimethylacetamide and acetonitrile. Following this, the cake was slurried for a period with warm acetonitrile (4O0C), filtered, washed with more acetonitrile and then dried (in vacuo or under a stream of nitrogen) to afford the anhydrous 2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}- quinazolin-4-yl)amino]-lH-pyrazol-5-yl}- N-(3-fluorophenyl)acetamide as an off-white solid in a yield of -90percent . 1H-NMR (DMSO d6): 10.55 (s, IH), 9.45 (br s, IH), 8.98 (s, IH), 8.8 (d, IH), 7.63 (pr of m, IH), 7.47 (pr of d, IH), 7.37 (m, 2H), 7.32 (d, IH), 6.9 (m, IH), 6.77 (s, IH), 4.32 (t, 2H), 3.83 (br s, 2H), 3.76 (t, 2H), 3.35 (m, 2H), 3.25 (m, 4H), 2.25 (m, 2H), 1.25 (t, 3H): MS (+ve ESI) : 508.4 (M+H)+.; 2-(3-[7-(3-Chloropropoxy)quinazolin-4-yl]amino}-lH-pyrazol-5-yl)-N-(3- fluorophenyl)acetamide and 2-(ethylamino)ethanol (ideally 12 molar equivalents) were added to N.N-dimethylacetamide under an inert atmosphere (such as provided by nitrogen) and the mixture heated to 90°C with stirring. After a period of 12 - 16 hours (ideally 12 hours) the reaction is cooled back to about 850C and water added in a controlled manner to maintain the temperature between 80 - 85°C. The batch is adjusted to 8O0C and seeded with crystals of the preferred form of the product (ideally an amount of about 1percent of the expected yield). The mixture was cooled to 200C in a carefully controlled manner over a period of about 20 hours so as to crystallise the product in the required form and of a size sufficient to afford a good filtration rate. The product is then filtered and washed with a mixture of water / N,N-dimethylacetamide and acetonitrile and suitably deliquored to afford a hydrated form of the product. Following this, the cake is slurried in situ for a period (ideally 2 hours) with warm acetonitrile (ideally at a temperature of 4O0C) then filtered, washed with more acetonitrile and then dried (in vacuo or under a stream of nitrogen) to afford the almost anhydrous 2-{3-[(7-{3-[ethyl(2- hydroxyethyl)amino]propoxy} -quinazolin-4-yl)amino]- lH-pyrazol-5-yl} - N-(3- fluorophenyl)acetamide as an off-white solid in a yield of 85 - 90percent. 1H-NMR (DMSO d6): 10.55 (s, IH), 9.45 (br s, IH), 8.98 (s, IH), 8.8 (d, IH), 7.63 (pr of m, IH), 7.47 (pr of d, IH), 7.37 (m, 2H), 7.32 (d, IH), 6.9 (m, IH), 6.77 (s, IH), 4.32 (t, 2H), 3.83 (br s, 2H), 3.76 (t, 2H), 3.35 (m, 2H), 3.25 (m, 4H), 2.25 (m, 2H), 1.25 (t, 3H): MS (+ve ESI) : 508.4 (M+H)+. |
| 85 - 90% |
In N,N-dimethyl acetamide; acetonitrile; at 20 - 90℃; for 32 - 36h;Product distribution / selectivity; |
2-(3-[7-(3-Chloropropoxy)quinazolin-4-yl]amino}-lH-pyrazol-5-yl)-N-(3- fluorophenyl)acetamide and 2-(ethylamino)ethanol (ideally 12 molar equivalents) were added to N,N-dimethylacetamide under an inert atmosphere (such as provided by nitrogen) and the mixture heated to 900C with stirring. After a period of 12 - 16 hours (ideally 12 hours) the reaction is cooled back to about 850C and water added in a controlled manner to maintain the temperature between 80 - 85°C. The batch is adjusted to 8O0C and seeded with crystals of the preferred form of the product (ideally an amount of about 1percent of the expected yield). The mixture was cooled to 2O0C in a carefully controlled manner over a period of about 20 hours so as to crystallise the product in the required form and of a size sufficient to afford a good filtration rate. The product is then filtered and washed with a mixture of water / N,N-dimethylacetamide and acetonitrile and suitably deliquored to afford a hydrated form of the product. Following this, the cake is slurried in situ for a period (ideally 2 hours) with warm acetonitrile (ideally at a temperature of 4O0C) then filtered, washed with more acetonitrile and then dried (in vacuo or under a stream of nitrogen) to afford the almost anhydrous 2-{3-[(7-{3-[ethyl(2- <n="27"/>hydroxyethyl)amino]propoxy}-quinazolin-4-yl)amino]-lH-pyrazol-5-yl}- N-(3- fluorophenyl)acetamide as an off-white solid in a yield of 85 - 90percent . 1H-NMR (DMSO d6): 10.55 (s, IH)5 9.45 (br s, IH), 8.98 (s, IH), 8.8 (d, IH), 7.63 (pr of m, IH), 7.47 (pr of d, IH), 7.37 (m, 2H), 7.32 (d, IH), 6.9 (m, IH), 6.77 (s, IH), 4.32 (t, 2H), 3.83 (br s, 2H), 3.76 (t, 2H), 3.35 (m, 2H), 3.25 (m, 4H), 2.25 (m, 2H), 1.25 (t, 3H): MS (+ve ESI) : 508.4 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +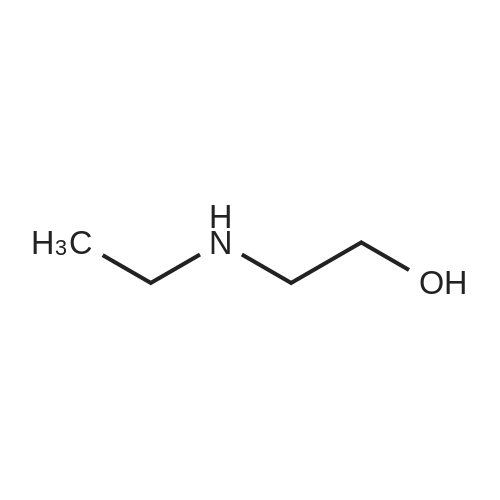

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping