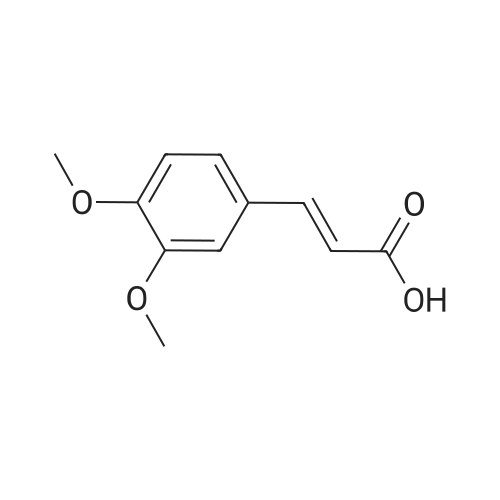
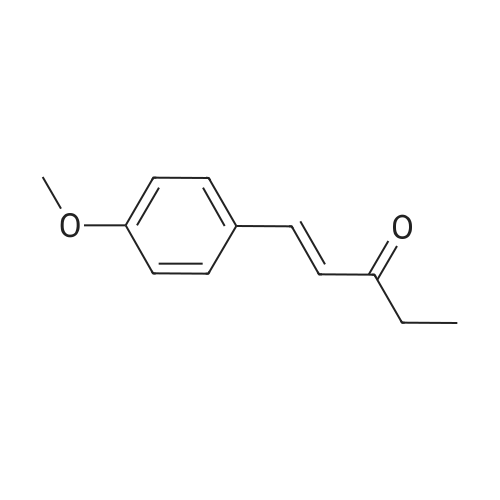
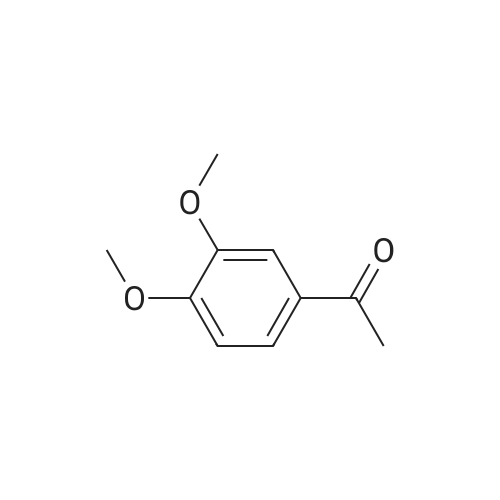
| 85% |
With sodium hydroxide; at 20℃; for 3h; |
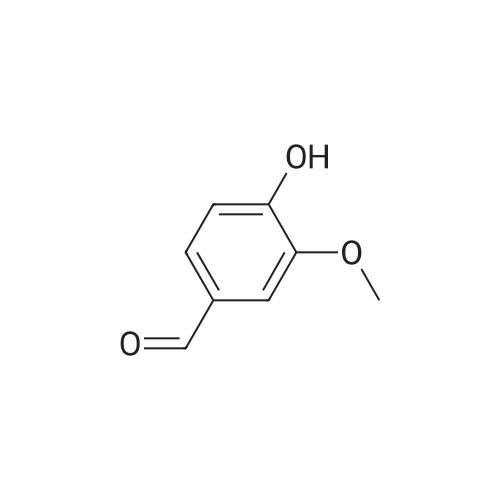
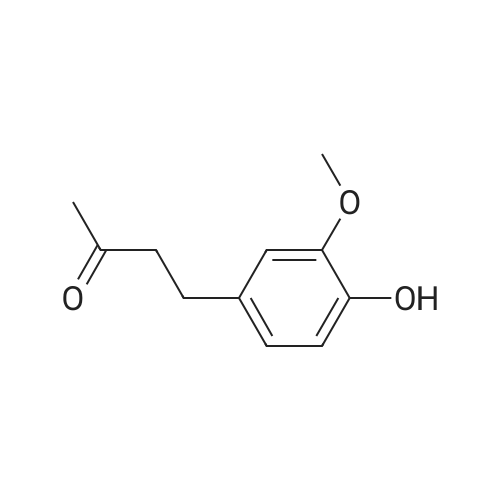
4.1.2. General procedure for the preparation of compounds (1f,g). Toa stirred solution of p-hydroxybenzaldehydes (10 mmol) in anhydrousacetone (15 mL) was added 10% NaOH solution (25 mL), andthe mixture was stirred for 3 h at room temperature. Acidification using 10% HCl until the color change of Congo red paper from red toblue. The resulting mixture was extracted with dichloromethane(30 mL3). The organic layers werewashed with brine (20 mL) and dried over MgSO4 were filtered, evaporated, and the residue waspurified by column chromatography (ethyl acetate/n-hexane1:10)to give pure 1f-g. |
| 80% |
With sodium hydroxide; In methanol; at 20℃; |
General procedure: For the preparation of analog 4, vanillin (5.6 mmol) wasused in a similar procedure as for compounds 2, followed bythe addition of an excess of acetone (3.0 cm3) to inhibit theformation of compound 2a and to furnish the expected intermediate4 for further use. Compound 4 (1.5 mmol) was firstdissolved in methanol, followed by the addition of indole-3-carboxaldehyde (1.7 mmol) to the solution. Sequentially,saturated NaOH in methanol was added dropwise, and themixture was stirred at room temperature. After 1 day, thereaction was neutralized and concentrated under reduced pressure, followed by extraction with EtOAc. The combinedorganic layers were washed with 30 cm3 brine and dried overanhydrous MgSO4,filtered, and concentrated under reducedpressure. Finally, the crude mixture was further purified byreversed-phase (C18) column chromatography in order toobtain indole derivative 5. |
| 72% |
With potassium hydroxide; at 20℃; for 2h; |
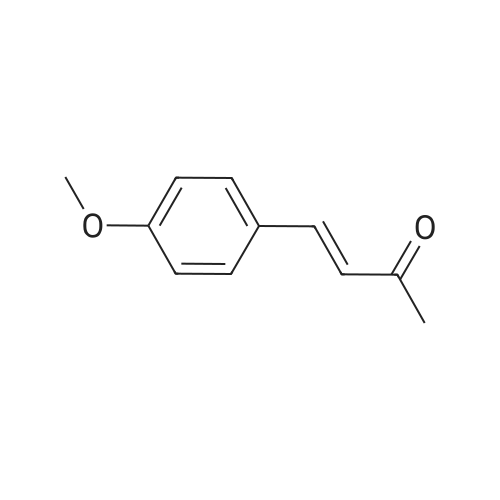
Vanillin (20 g) was dissolved in acetone (50 mL) in a round bottomflask and 40% KOH (5 mL) was added to this reaction mixture.It was kept on stirring at room temperature. After the completionof reaction (monitored by TLC), reaction mixture was acidified withdilute hydrochloric acid until pH 7 was achieved. It was thenpoured in ice-cold water, filtered and dried to get the desired product,that is, vinyldenacetone (VDA). The physical data of vinyldenacetoneis given below:4.2.1. (E)-4-(4-Hydroxy-3-methoxyphenyl)but-en-2-one (VDA)Yield 72%; mp 71-73 C. 1H NMR (CDCl3, 500 MHz, d, TMS = 0):2.36 (s, 3H), 3.91 (s, 3H), 6.58 (d, J = 16.5 Hz, 1H), 6.92 (d, J = 7.5 Hz,1H), 7.04-7.08 (m, 2H), 7.45 (d, J = 16.5 Hz, 1H). 13C NMR (CDCl3,125 MHz, d, TMS = 0): 27.22, 55.84, 109.56, 114.99, 123.52,124.82, 126.79, 144.03, 147.08, 148.51, 198.72. |
| 72% |
With potassium hydroxide; In water; at 20℃; for 2h; |
Vanillin (20g) was dissolved in acetone (50ml) in a round bottom flask and 40% KOH (5ml) was added to this reaction mixture. It was kept on stirring at room temperature. After the completion of reaction (monitored by TLC), reaction mixture was acidified with dilute hydrochloric acid until pH 7 was achieved. It was then poured in ice-cold water, filtered and dried to get the desired product i.e. vinyldenacetone (VDA). The physical data of vinyldenacetone is given below. Yield 72%; mp 71-73C. 1H NMR (CDCl3, 500MHz, delta, TMS=0): 2.36 (s, 3H), 3.91 (s, 3H), 6.58 (d, J=16.5Hz, 1H), 6.92 (d, J=7.5Hz, 1H), 7.04-7.08 (m, 2H), 7.45 (d, J=16.5Hz, 1H). 13C NMR (CDCl3, 125MHz, delta, TMS=0): 27.22, 55.84, 109.56, 114.99, 123.52, 124.82, 126.79, 144.03, 147.08, 148.51, 198.72 |
|
With sodium hydroxide; In water; ethyl acetate; |
A second series of compounds 6a-6c, 6f, 6g, 6j-6q were synthesized containing two aryl rings separated by a five carbon unsaturated spacer having a single carbonyl. These compounds were designed to test the importance the length of the spacer and the number of carbonyls in the spacer. The synthesis of compounds 6a-6c, 6f, 6g, 6j-6q involves a base catalyzed aldol reaction with acetone and substituted benzaldehydes as depicted in FIG. 3. Two additional compounds, 6r and 6s, having a five carbon spacer contain two different aryl rings. These compounds were designed to test the importance of symmetry in compounds with a five carbon spacer. As depicted in FIG. 3, these compounds were synthesized using consecutive base catalyzed aldol reactions as described by Masuda (Masuda, T., Jitoe, A., Isobe. J., Nakatani, N., Yonemori, S. 1993. Anti-Oxidative and Anti-inflammatory Curcumin-Related Phenolics from Rhizomes of Curcuma Domestica. 32:1557-1560). Two additional compounds 7a and 7f contain a single aryl ring and a 4-carbon unsaturated chain with a carbonyl These compounds were designed to test the importance of the necessity of two aryl rings. Compounds 7a and 7f were synthesized as depicted in FIG. 3 using a base catalyzed aldol reaction with excess acetone and substituted benzaldehydes. Two compounds, 8b and 9b, were synthesized as shown in FIG. 3. These compounds contain a saturated five carbon spacer. Compound 9b has a hydroxyl group on the spacer rather than a carbonyl. These compounds were designed to test the importance of unsaturation and the necessity of a carbonyl in the spacer. The synthesis of these compounds was performed by reacting compound 6b with palladium on activated charcoal under a hydrogen atmosphere to give a mixture of compounds 8b and 9b, which were separated by chromatography. Compounds 9a-9v contain two identical aryl rings separated by an unsaturated five carbon spacer having a single carbonyl whereas compounds 9x and 9y have two different aryl rings. These compounds were designed to test the importance of the length of the spacer between the two aryl rings. Compounds 9a-9w were prepared from acetone and a substituted benzaldehyde in a base catalyzed aldol reaction as described by Masuda. In the case of phenolic benzaldehydes, the phenol was protected with a methoxymethyl group prior to the aldol reaction and deprotected later to give the free phenol. Compounds 9u and 9v were prepared from 9a and 9r respectively by reaction with acetic anhydride as described by Ali. Compounds 9x and 9y were prepared using two consecutive base catalyzed aldol reactions. |
|
With sodium hydroxide; In water; at 20℃; |
25 ml 20% aq. sodium hydroxide was added to the acetone (30 ml) solution of vanillin (5.0 g, 32.89 mmol) and then this was stirred overnight at room temperature. The reaction mixture was diluted with ice water and 35 ml conc. HCl was added, a brown precipitate formed which was filtered, washed with cold water and dried to obtain (E)-4-(4'-hydroxy-3'-methoxyphenyl)-3-buten-2-one. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping