|
|
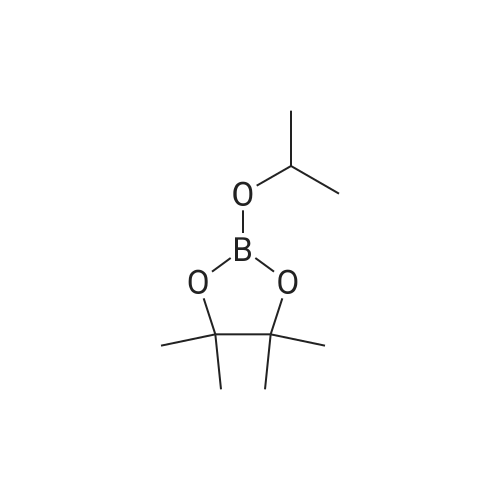
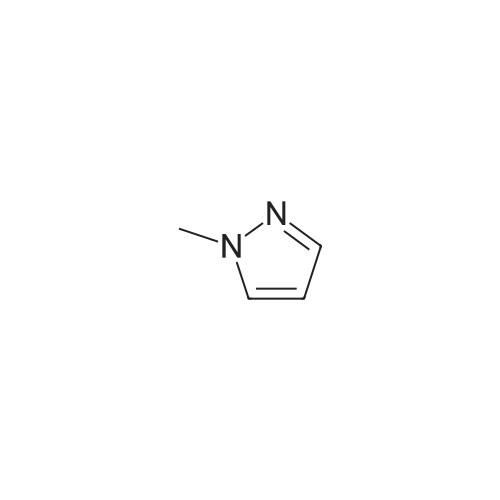
To a suspension of NaH (60% in mineral oil, 3.5 g, 146 mmol, washed with 200 mL of hexane) in THF (200 mL) was added 4-methyl-1 H-pyrazole (10 g, 122 mmol) at 0 0C dropwise. After stirring at RT for 1 h, to above suspension was added MeI (7.3 mL, 117 mmol) dropwise at 0 0C. The reaction mixture was stirred overnight. The NaI by-product was removed by filtration and the filtrate solution was used directly in the next step.At 00C, to above THF solution of 1 ,4-dimethyl pyrazole was added n-BuLi (2.5M in hexane, 58.5 mL, 146 mmole). The reaction solution was stirred for 2 hour at RT and then cooled to -78C [J. Heterocyclic Chem. 41 , 931 (2004)]. To the reaction solution was added 2-isopropoxy-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane (27.2 g, 146 mmole). After 15 min at -78C, the reaction was allowed to warm to 00C and stir for 3h. The reaction was diluted with saturated NH4CI solution and extracted with DCM. The organics were dried over Na2SO4 and concentrated under vacuum to afford the title compound as a brown solid (21 g, 78%) which was used directly without further purification: LC-MS: 141 (M-C6H12)"1", 223 (M+H)+. 1H NMR (CDCI3): delta 7.28 (s, 1 H), 4.03 (s, 3H), 2.22 (s, 3H), and 1.32 (s, 12H). |
| 142 mg |
|
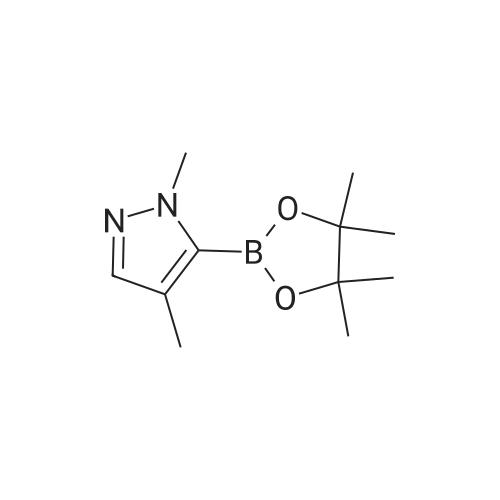
1,4-dimethyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole A solution of 1,4-dimethyl-1H-pyrazole (480.0 mg, 4.993 mol) in tetrahydrofuran (20 mL, 300 mmol) at 0 C. was added 1.6 M n-butyllithium in hexane (4.7 mL, 7.5 mmol). The solution was stirred at room temperature for 1 h and then cooled to -78 C. To the solution was added 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1.63 mL, 7.99 mmol). The reaction mixture was stirred at -78 C. for 0.5 h, then warmed up to 0 C. (taking 0.5 h). The reaction was quenched with brine and extracted with EtOAc (3*). The combined organic phases were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by combi-flash chromatography and eluted with EtOAc/hexane (0-60%). The purification gave 142 mg of product as white solid. |
|
|
Step 1. 1, 4-Dimethyl-5-( 4, 4, 5, 5-tetrame orolan-2-yl)-lH-pyrazole l,4-Dimethyl-7H-pyrazole (50 mg, 0.5 mmol) was stirred in THF (2 mL) and cooled to 0 C. A solution of 1.6 M w-butyllithium in hexanes (390 mL) was added dropwise by syringe and the mixture was allowed to warm to room temperature for 2 h. The mixture was cooled to -78 C and 2-isopropoxy-4,4,5,5-tetramethyl-l,3,2-dioxaborolane(110 mL, 0.52 mmol) was added dropwise by syringe. The mixture was stirred at -78 C for 15 min. and at 0 C for 3 h. The mixture was diluted with EtOAc and washed with brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. Purification by chromatography on silica gel using EtOAc in hexanes gave the sub-title compound. LCMS calc. for CnH2oB 202 (M+H)+: m/z = 223.2; found: 223.0. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping