|
|
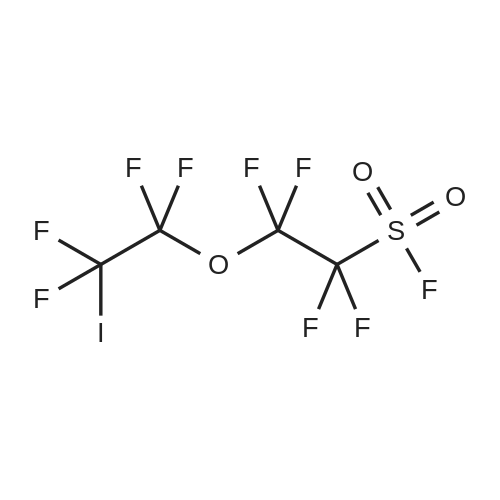
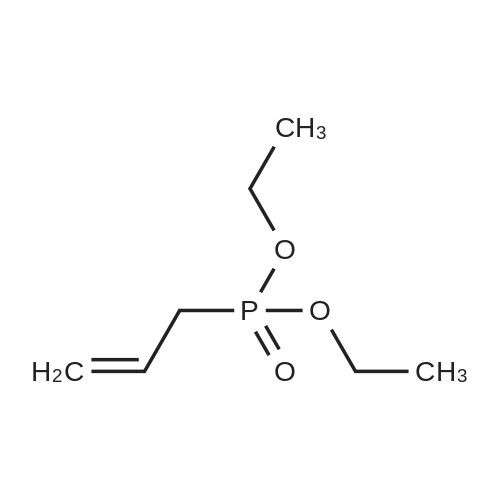
Example 3: (ETO) 20PCH2CH2CH2CF2CF20CF2CF2SO2F was prepared using the following procedure: A mixture of 110.6 g of diethyl allylphosphonate, 277.6 g of ICF2CF2OCF2CF2S02F was heated to 80 to 85 C under N2. 0. 5 g of benzoyl peroxide was added and the reaction mixture was stirred for 1.5 hr. Additional 0.5 g of benzoyl peroxide was added and the mixture was stirred for 2 hr and these steps were repeated four more times. GC indicated no starting materials. The mixture was evaporated to remove excess diethyl allylphosphonate. 120 mL of BU3SNH were added and the mixture was stirred until no starting material remained. It was then diluted with ether, and treated with aqueous KF solution to remove BU3SNL, washed with water and dried over MGSO4. After removal of the ether, 159.3 g of product were obtained. 19F NMR: +44.4 (s, 1F),-83. 7 (m, 2F), - 88. 9 (m, 2F),-113. 2 (s, 2F),-118. 8 (m, 2F). HRMS: Calcd for C11 H1606FGP1 S1 : 479.03398 : Found: 479.03253. (HO) 20PCH2CH2CH2CF2CF2OCF2CF2S02F was prepared using the following procedure: A mixture of 46.9 g of (ETO) 20PCH2CH2CH2CF2CF2OCF2CF2S02F and conc. 200 mL of concentrated HCI was heated at 110 C for 4 days and evaporated to remove volatile to give product. 19F NMR: +44.7 (s, 1 F),-83. 3 (m, 2F), - 87. 7 (M, 2F), -112. 9 (S, 2F), -118. 3 (M, 2F). (HO) 20PCH2CH2CH2CF2CF20CF2CF2SO3H was prepared using the following procedure: A mixture of 10.0 g of (ETO) 20PCH2CH2CH2CF2CF2OCF2CF2S02F and 2.5 g of LIOH in 60 mL of MEOH was stirred overnight and then filtered and evaporated to give solids, which were boiled with dry CH3CN (3X60 mL), filtered and the filtrates were evaporated to give 10.1 g of product. 19F NMR indicated no sulfonyl fluoride peak. The solids were refluxed with 80 mL of conc. HCI for 2 days. After removal of volatiles, 8.2 grams of residue were obtained. P 19F NMR:-82. 9 (M, 2F), -88. 5 (S, 2F), -118. 0 (M, 2F), -118. 5 (S, 2F). 7.2 G of solids were dissolved in water and run through an ion exchange column at two drops per minute and then evaporated and dried at 100 C in full vacuum to give 6.65 g of wax solids. Zr [03PCH2CH2CH2CF2CF20CF2CF2SO3H] 2 was prepared using the following procedure: 0.39 g (1.2 MMOL) of ZROCI2/8H2O was dissolved in 5 mL of water and then poured in to a solution of 1.0 g (2.38 MMOL) of (HO) 20PCH2CH2CH2CF2CF20CF2CF2SO3H in 5 mL of water. After being stirred at 70-80 C for 20 hours, the mixture was then heated in a vacuum oven at 85 C for 54 hrs and at 110 C for 6 hrs. 1.03 g of brown solid were obtained, that were ground into a fine powder, and pressed into pellets. Conductivity, measured using the procedure described above, was found to be 15.10 mS/cm at 150 C. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping