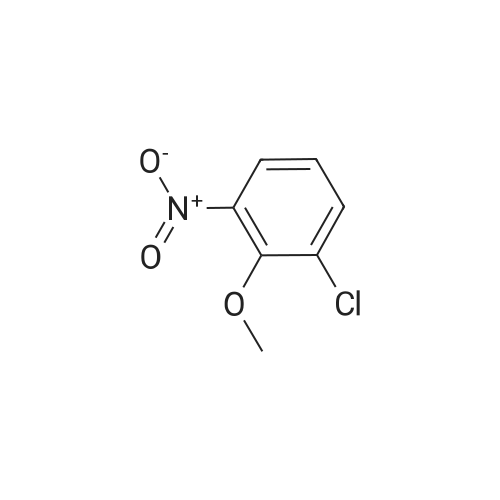
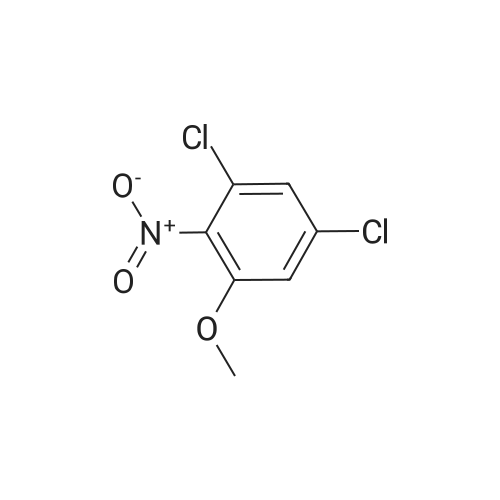
| 82% |
With caesium carbonate; at 60℃; |
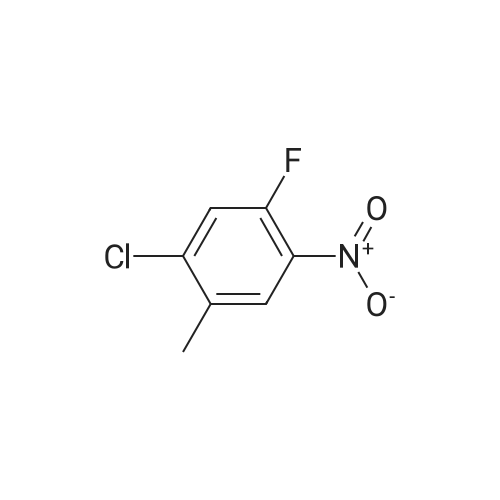
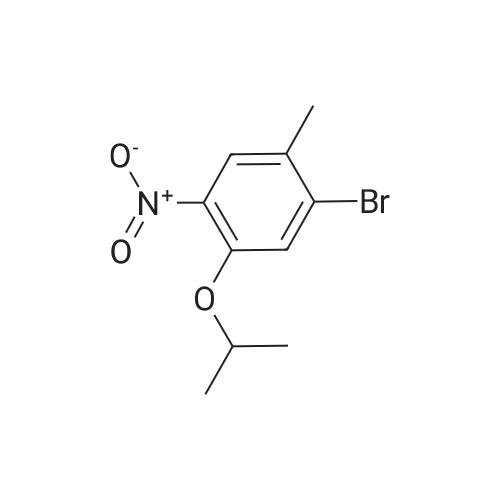
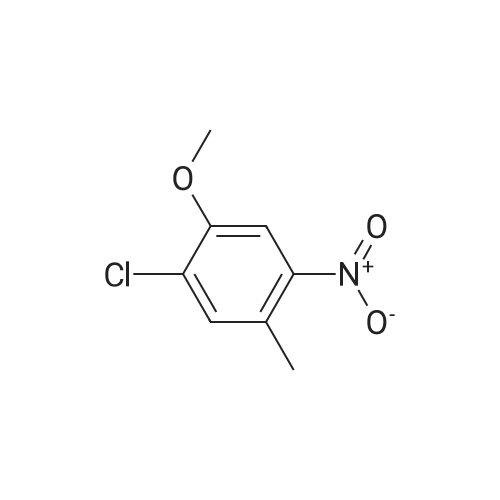
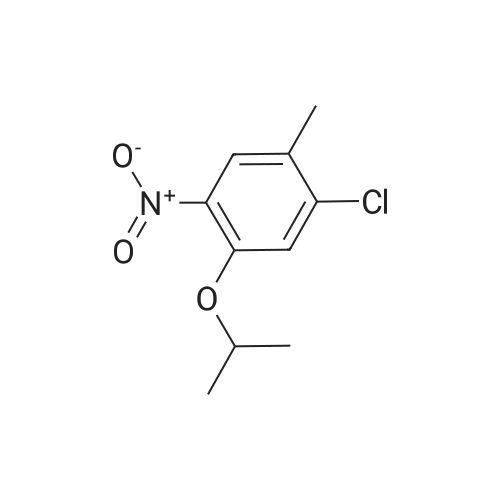
The title compound <strong>[112108-73-3]2-chloro-4-fluoro-5-nitrotoluene</strong> (i.e., Compound 2-1, 14.2 g, 74.9 mmol) was dissolved in isopropanol (100.0 mL), cesium carbonate (122.0 g, 374.4 mmol) The reaction mixture was concentrated at 60 C overnight. After completion of the reaction, the mixture was concentrated to remove isopropanol, poured into 500.0 mL of water, extracted with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate and concentrated to give Compound 2-2 (14.3 g), yield: 82.0%. |
| 74.4% |
With caesium carbonate; at 60℃; for 24.0h; |
1-Chloro-5-fluoro-2-methyl-4-nitrobenzene (3.260 g, 17.197 mmol)Cesium carbonate (28.015 g, 85.984 mmol) was added to a solution ofPropanol (35 mL) at 60 C was stirred at the same temperature for 24 hours, and then the temperature was lowered to room temperature to terminate the reaction. The reaction mixture was filtered through a paper filter to remove the solid. The solvent was removed from the filtrate under reduced pressure, water was poured into the resulting concentrate, and the mixture was extracted with ethyl acetate. The organic layer was washed with a saturated aqueous sodium chloride solution,The water was removed with magnesium sulfate, filtered and concentrated under reduced pressure.The concentrate was purified by column chromatography (SiO2, 40 g cartridge; ethyl acetate / hexane = 5%) and concentrated to obtain 2.938 g (74.4%) of 1-chloro-5-isopropoxy-2-methyl-4-nitrobenzene As a light brown solid. |
|
With caesium carbonate; at 60℃; |
To a solution of 25 g (0.131 mol) of <strong>[112108-73-3]2-chloro-4-fluoro-5-nitrotoluene</strong> in 250 ml of 2- propanol is added 208 g (0.659 mol, 5 eq.) of Cs2CO3. The mixture is stirred at 6O0C overnight and most of the 2-propanol is evaporated under reduced pressure. Water is added and the solution is extracted with EtOAc. The organic layers are combined, dried over MgSO4, concentrated and the crude product filtrated over a silica plug (eluent: 95/5 hexanes/EtOAc) to afford 2-chloro-4-/,sopropoxy-5-nitrotoluene as a pale yellow fluffy solid. |
|
With potassium carbonate; for 40.0h;Reflux; |
2-chloro-4-fluoro-5-nitro-toluene was dissolved in 1200 ml of isopropanol and 2 L of a three-necked flask was added. Add 429 g of anhydrous potassium carbonate powder. Stir under the temperature to reflux. The reaction was kept under reflux for ~ 40 hours. Concentrate to remove most of the isopropanol. Add 2L of water, And extracted twice with ethyl acetate. The ethyl acetate layer was combined, Washed. Concentrated ethyl acetate, To give a brown 2-chloro-4-isopropoxy _5_ nitro - toluene. |
|
With potassium carbonate; for 40.0h;Reflux; |
EXAMPLE 2 Preparation of 2-chloro-4-isopropyoxy-5-nitrotoluene <strong>[112108-73-3]2-chloro-4-fluoro-5-nitrotoluene</strong> was dissolved in 1200 ml of isopropanol and the solution was added in a 2 L of a three-necked flask. 429 g of anhydrous potassium carbonate powder was added therein. The mixture was heated to reflux under stirring. The reaction was conducted for about 40 hours under reflux. Most of the isopropanol was removed via concentration. 2 L of water was added and the mixture was extracted with ethyl acetate twice. The ethyl acetate layer was combined and washed with water. Ethyl acetate layer was concentrated to give 2-chloro-4-isopropoxy-5-nitrotoluene as a brown substance. H NMR Data 1H NMR (CDCl3): delta7.71(s, 1H), 7.07(s, 1H), 4.61(m, 1H), 2.34(s, 3H), 1.40(d, 3.2, 6H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping