| 83% |
With water; sodium hydroxide; In methanol; at 25 - 30℃; |
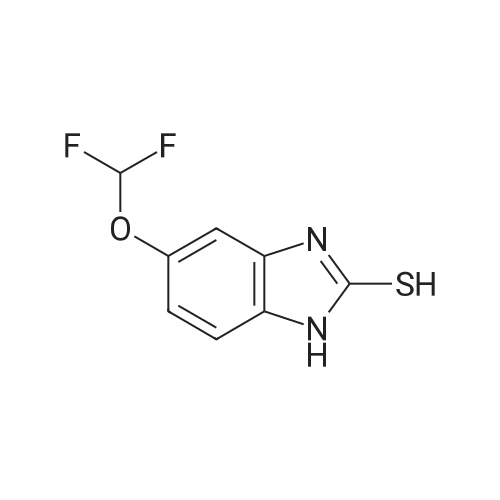
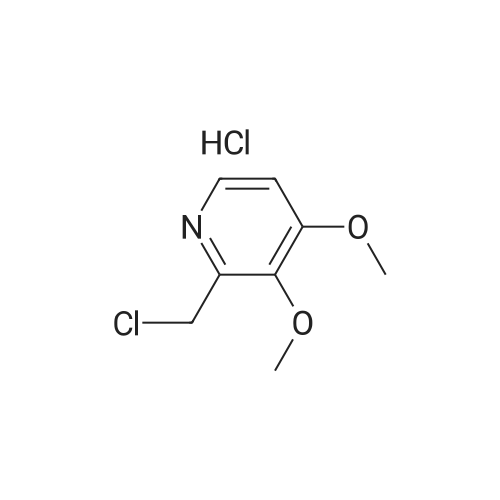
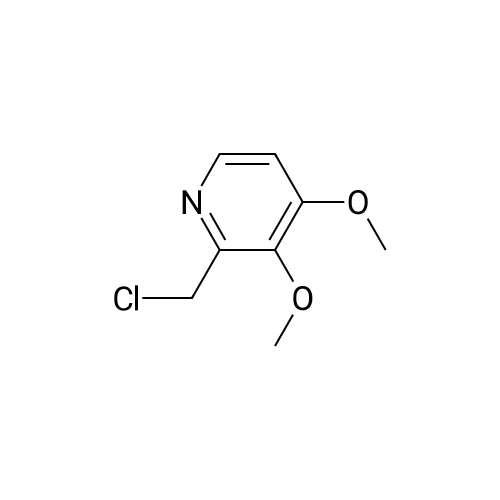
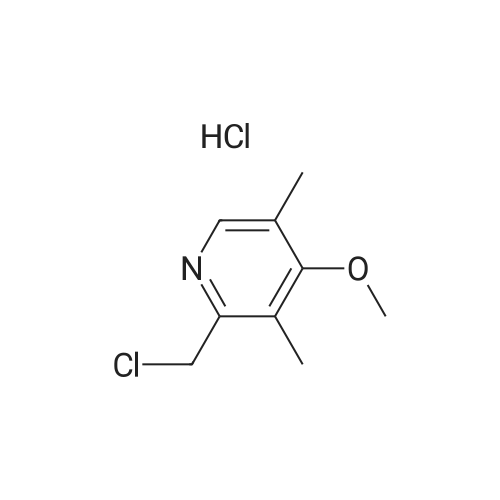
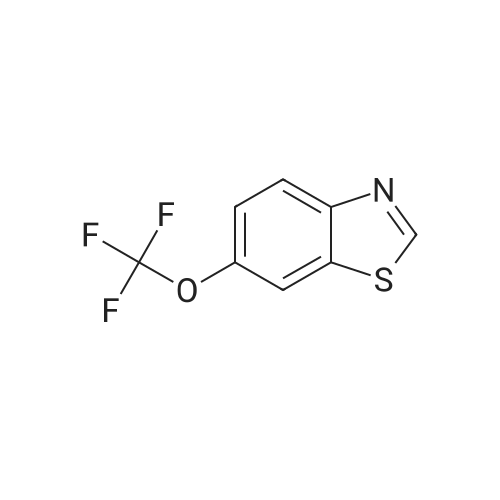
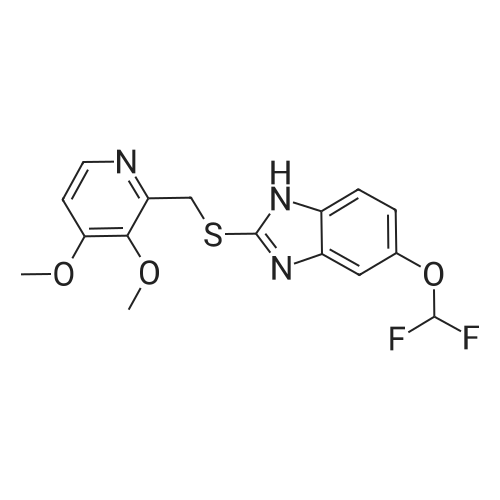
Methanol (270 ml) was added to a solution of NaOH (41.5 gms) in water (180 ml), followed by addition of <strong>[97963-62-7]5-difluoromethoxy-2-mercapto-1H-benzimidazole</strong> (105.2 gms). A solution of 2-chloromethyl-3,4-dimethoxy-pyridine.hydrochloride (100.3 gm in water (150 ml)) was gradually added to the reaction mixture and stirred at 25-30 C. till completion of the reaction. After completion, as monitored by TLC, the reaction mixture was filtered and the obtained solid was dried to give compound IV-A-11.Yield: 140.6 gm (83%).1H NMR (400 MHz, CDCl3): delta 8.27 (d, J=5.6 Hz, 1H), 7.48 (d, J=8.8 Hz, 1H), 7.32 (d, J=2 Hz, 1H), 6.99 (dd, J=2.4, 8.8 Hz, 1H), 6.87 (d, J=5.6 Hz, 1H), 6.50 (t, J=74.8 Hz, 1H), 4.39 (s, 2H), 3.95 (s, 3H), 3.93 (s, 3H).ESI-MS: 368.9 (M+1). |
|
With sodium hydroxide; In ethanol; water; at 20℃; for 5h; |
Reaction vessel was charged with 1050 kg water, 1050 li- ters of ethyl alcohol and 15.2 kg of sodium hydroxide flakes. 19.5 kg of 2-mercapto-5-difluoromethoxybenzimidazole was added to the vessel followed by addition of 20 kg 2-chloromethyl-3,4- dimethoxypyridine. HCl. The mixture was stirred at room tempera- ture for 4 hours. HPLC analysis should confirm the total con- sumption of 2-chloromethyl-3,4-dimethoxypyridine. HCl. 500 li- ters of water were added to the vessel and the mixture was stirred for additional 1 hour at room temperature. Mixture was centrifuged. Centrifuge was first washed with 500 liters of 0,1 N sodium hydroxide and then with plenty of water till the wash water was neutral to litmus. Wet cake was charged another vessel containing 500 liters of methylene chloride and stirred till a clear solution was obtained. 200 liters of water were added to the vessel and stirred for 30 minutes. Organic phase was decanted, filtered over celite and transferred to a vessel equipped with brine cooling system. 26 kg of sulfide product on dry basis was ob- tained from this step. |
|
With sodium hydroxide; In water; at 25 - 35℃; for 3h;Product distribution / selectivity; |
Example 1: Preparation of Pantoprazole sodium compound of formula-la:Added a solution of 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (50 grams in 250ml of water) to a solution of 49.8 grams of 5-difluoromethoxy 2-mercaptobenzimidazole, 500 ml water and sodium hydroxide (22.5 grams of flakes in 27.5 ml of water), slowly at 25-35C. Stirred the reaction mixture for 3 hours. Extracted the reaction mixture thrice with methylene chloride. Separated the organic and aqueous layer. Washed the organic layer with water. Cooled the organic layer to -5 to 0C. Added 550 grams of 3.1% sodium hypochlorite solution having pH 8.75 and assay 3.2 to the above reaction mixture at -5 to 0C. Stirred the reaction mixture for 3 hours at -5 to 0C. Quenched the reaction mixture with 56 grams of ammonium sulphate at below 10C. Stirred the reaction mixture for 30 minutes. Separated the organic and aqueous phases. Extracted the aqueous phase twice with methylene chloride. Washed the organic layer with water. Dried the organic phase over sodium sulphate. Distilled the solvent completely under reduced pressure at below 45C. Added 37.5 ml of acetone to the above crude and distilled the solvent completely under reduced pressure at below 45C. Dissolved the residue in 375 ml of acetone at 25-35C. Heated the reaction mixture to reflux temperature. Stirred the reaction mixture for 30 minutes at reflux temperature. Cooled the reaction mixture to 18-23C. Added aqueous sodium hydroxide solution (8.5 grams in 10 ml of water) at 18-23C. Stirred the reaction mixture for 1 hour at 18-23C. Cooled the reaction mixture to 0-5C. Stirred the reaction mixture for 3 hours. Filtered the solid and washed with acetone followed by washed with methylene chloride. The obtained solid is purified in acetone to get pure compound.The amount of sulfone compound of formula-6 and compound of formula- 1 present in the obtained solid was measured using HPLC and the results are as follows. <n="19"/>Yield: 65 grams Example-3: Preparation of pantoprazole sodium sesquihydrate compound of formula-la:Added a solution of 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (50 grams in 250ml of water) to a solution of 49.8 grams of 5-difluoromethoxy-2- mercaptobenzimidazole, 500 ml of water and aqueous sodium hydroxide (22.5 grams of flakes in 27.5ml of water), slowly at 25-350C. Stirred the reaction mixture for 3 hours. Extracted the reaction mixture thrice with methylene chloride. Separated the organic and aqueous layer. Washed the organic layer with water. Added 550 grams of 3.1% sodium hypochlorite having pH 8.75 and assay 3.2 to the above reaction mixture at 25-300C for 2 hours. Stirred the reaction mixture for 10 hours at 25-3O0C. Quenched the reaction mixture with water at 25-300C. Stirred the reaction mixture for 30 minutes at 25-300C. Separated the organic and aqueous phases. Extracted the aqueous layer with methylene chloride. Washed the organic phase twice with aqueous sodium hydroxide solution. Separated the <n="20"/>phases. Cooled the aqueous layer to 10-150C. Adjusted the pH of the reaction mixture to 9.3 with aqueous acetic acid. Added 250 ml of methylene chloride. Stirred the reaction mixture for 15 minutes. Separated the organic phase. Extracted the reaction mixture with methylene chloride. Washed the organic layer with water. Distilled the solvent completely from organic layer at below 45C under reduced pressure. Added 37.5 ml of acetone to the crude and distilled the solvent completely under reduced pressure at below 45C. Dissolved the residue in 375 ml of acetone at 25-35C. Heated the reaction mixture to reflux temperature. Stirred the reaction mixture for 30 minutes at reflux temperature. Cooled the reaction mixture to 18-23C. Added aqueous sodium hydroxide solution (8.5 grams in 10 ml of water) at 18-23C. Stirred the reaction mixture for 1 hour at 18-23C. Cooled the reaction mixture to 0-50C and 35 ml of methylene chloride was added. Stirred the reaction mixture for 3 hours. Filtered the solid and washed with methylene chloride. The above obtained compound can optionally purified as follows.Acetone (400 ml) was added to the above obtained wet compound and heated to reflux. The obtained solution was treated with carbon and cooled the filtrate to 0-5C. Stirred for 2 hours. Filtered the precipitated solid and washed with 30 ml of chilled acetone followed by washing with 50 ml of methylene chloride. Methylene chloride (250 ml) was added to the obtained wet compound at 25-35C. Stirred the reaction mixture for 90 minutes at 25-350C. Filtered the precipitated solid and washed with 25 ml of methylene chloride. Dried the compound at 40-500C for 10 hours.Yield: 70 grams W.C : 5.9 %HPLC : 99.93 %; 0.02 % (Sulfone Impurity) PSD : before micronization : D (v, 0.1) is 0.4 mum ; D (v, 0.5) is 8.73 mum; D (v, 0.9) is 27.7 mum andD(4,3) is 12.02 mum.PSD : after micronization :D (v,0.1) is 1.75 mum; D (v,Q.5) is 4.92 mum; D (v,0.9) is 13.10 mum andD(4,3) is 6.35 mum.Exam... |
|
With sodium hydroxide; In water; at 20℃; for 4 - 5h; |
Example 1 : Preparation of Pantoprazole Sodium2-Mercapto-5-difluoromethoxy benzimidazole (50 g) was added to an aqueous solution of sodium hydroxide (21.3 g in 350 mL de-ionized water) at room temperature to obtain a clear solution. An aqueous solution of 2-chloromethyl-3, 4-dimethoxypyridine hydrochloride (50 g in 150 mL water) was added to the above solution over a period of about 2.0-2.5 hours. The reaction mixture was stirred vigorously for about 2-2.5 hours. Progress of the reaction was monitored by thin-layer chromatography. The reaction mixture was extracted with dichloromethane and washed with water. Organic layer was concentrated. Methanol (50 mL) was added to the organic layer. The reaction mixture was cooled to -5 to -200C. Aqueous solution of sodium hydroxide (11.8 g in 50 mL water) was added followed by addition of sodium hypochlorite solution (431 mL) in an aqueous solution of sodium hydroxide (20 g/ 100 mL) over a period of about 30 to about 45 minutes. The progress of the reaction was monitored by thin-layer chromatography. After completion of the reaction, the reaction mixture was quenched with 5% sodium hydrogen sulphite solution (500 mL). Water (500 mL) was added. pH of the reaction mixture was adjusted to 9.0-10.5. Layers were separated and the aqueous layer was extracted with dichloromethane. The combined dichloromethane layers were concentrated completely to obtain a red-brown colored residue.The residue was dissolved in acetone (375 mL). The reaction mixture was cooled to 20-250C. Aqueous solution of sodium hydroxide (9.2 g in 25 mL water) was added followed by addition of a seed crystal of pantoprazole sodium. The reaction mixture was stirred, cooled, stirred, filtered and washed with cold acetone to obtain crude pantoprazole sodium as a wet cake. |
|
|
2-Mercapto-5-difluoromethoxy benzimidazole (50 g) was added to an aqueous solution of sodium hydroxide (21.3 g in 350 mL de-ionized water) at room temperature to obtain a clear solution. An aqueous solution of 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (50 g in 150 mL water) was added to the above solution over a period of about 2.0-2.5 hours. The reaction mixture was stirred vigorously for about 2-2.5 hours. Progress of the reaction was monitored by thin-layer chromatography. The reaction mixture was extracted with dichloromethane and washed with water. Organic layer was concentrated. Methanol (50 mL) was added to the organic layer. The reaction mixture was cooled to -5 to -20 C. Aqueous solution of sodium hydroxide (11.8 g in 50 mL water) was added followed by addition of sodium hypochlorite solution (431 mL) in an aqueous solution of sodium hydroxide (20 g/100 mL) over a period of about 30 to about 45 minutes. The progress of the reaction was monitored by thin-layer chromatography. After completion of the reaction, the reaction mixture was quenched with 5% sodium hydrogen sulphite solution (500 mL). Water (500 mL) was added. pH of the reaction mixture was adjusted to 9.0-10.5. Layers were separated and the aqueous layer was extracted with dichloromethane. The combined dichloromethane layers were concentrated completely to obtain a red-brown colored residue. The residue was dissolved in acetone (375 mL). The reaction mixture was cooled to 20-25 C. Aqueous solution of sodium hydroxide (9.2 g in 25 mL water) was added followed by addition of a seed crystal of pantoprazole sodium. The reaction mixture was stirred, cooled, stirred, filtered and washed with cold acetone to obtain crude pantoprazole sodium as a wet cake. |
|
|
Example 3 - Synthesis of 24(3,4-Dimethoxy)pyridme-2-vI)methylsulphinyl]-5- difluoromethoxy-lH-benzimidazole without isolation of sulphide To a solution of NaOH (46.59 g in 500 ml demineralised water) 5-difiuoromethoxy-2- mercaptobenzimidazole was added and the mixture obtained was stirred for 15 minutes for complete dissolution. Then 200 ml methanol was added and the mixture cooled to 15 0C. 2-chloromethyl-3,4-dimethoxypyridine hydrochloride was added slowly at 10 to 15 0C. The reaction mass was warmed up to 20 -300C and stirred for 6-8 hours. Then 600 ml methylene dichloride was added and the reaction mixture was cooled to 0 - 5 0C, followed by charging 2 g TBAB and 5 % sodium hypochlorite solution in water (602 ml) thereto, the latter slowly for 45 min. The temperature was raised to 10 - 15 0C and the reaction mass was stirred for 2-3 hours.5 % sodium thiosulphate solution (100 ml) was added and the mixture was stirred for 30 min. The methylene dichloride layer was separated and the aqueous layer was extracted with 2 X 200 ml methylene dichloride. The aqueous layer was charcoalised with 5% carbon, filtered and cooled. Then the pH was adjusted with 50 % acetic acid to 8.5, the gummy compound extracted with methylene dichloride 3 X 300 ml. The EPO <DP n="15"/>solvent was evaporated under vacuum at 35C and 300 ml ethyl acetate was added at RT. Then it was heated to 50-550C to dissolve the residue completely, cooled to 0-5 0C and maintained for 1.0 hour. The compound was filtered and washed with 2x 25 ml of acetonitrile. Drying was performed at 40 - 450C under vacuum for 2-3 hrs. The dry product thus obtained weighed 85 - 9O g (65% yield based on 2- chloromethyl-3,4-dimethoxypyridine hydrochloride, purity as determined by HPLC was 98.5 - 99 %. |
|
With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide; In dichloromethane; water; at 30 - 35℃; for 6.1h; |
A solution of water, 1L, sodium hydroxide (43 g, 1.13 mol) and tetrabutylammonium bisulfate (5.08 g, 0.015 mol) were added to a 2.5 L three-necked flask, stirred to clarification, cooled to room temperature, And <strong>[97963-62-7]5-difluoromethoxy-2-mercapto-1H-benzimidazole</strong> (100 g, 0.466 mol) were added and stirred to a solution of 2-chloromethyl 3,4-dimethoxypyridine hydrochloride (98.5 g , 0.44 mol) of water in 0.6 L solution, 1.6 h after the drop, 30 ~ 35 C reaction 4.5h, TLC [developing agent: ethyl acetate: methanol = 9: 1] detection reaction is completed after the separation, The chloroform layer was washed with O.lmol / L sodium hydroxide solution and then an aqueous solution of potassium tungstate(Na2W0.4H2O1818g, H200.76L), 30% hydrogen peroxide (260ml), weak acid pH of 3? 5, - 5 C_0 C for 4.5 hours, TLC (developing solvent: ethyl acetate: methanol = 9: 1) detection, the reaction is completed by adding saturated sodium carbonate solution adjusted PH value to neutral, standing stratification, take the chloroform layer, add 15gNa0H and 50ml distilled water preparation of alkaline solution, stirring at room temperature 1.5-2 hours, the detection reaction is completed , The reaction solution was cooled to 0 C to crystallize, filtered, and the filter cake was washed with 200 ml of cold acetone solution and dried in vacuo at 35-40 C to give 170.9 g of crude pantoprazole sodium. |
| 16.6 g |
With sodium hydroxide; In dichloromethane; at 20 - 30℃; for 2h; |
To the reaction flask was added 10 g of 2-chloromethyl-3,4-dimethoxypyridine hydrochloride(Compound 1), 10 g of <strong>[97963-62-7]5-difluoromethoxy-2-mercapto-1H-benzimidazole</strong>(Compound 2), 100 ml of methylene chloride was added, and 130 g of a 10% sodium hydroxide solution was added dropwise, and the mixture was stirred at 20 to 30 C for 2 hours, Static separation, dichloromethane layer washed twice, each time with water 30ml,And then distilled under reduced pressure to give 16.6 g of a yellow oil (Intermediate 3); |
|
With sodium hydroxide; In water; at 25 - 30℃; |
Add water (solvent) to the reaction kettle, add sodium hydroxide (neutralizer) in portions, stir to dissolve at 27-30 C, add the starting reactant III (starting material), stir at 27-30 C Dissolve, control the drop rate to add an aqueous solution of II (starting material) within 2.0 to 2.9 hours. The reaction of II with III is that the hydrogen on the sulfhydryl group on the starting material III is replaced by the starting material II while losing one molecule of hydrogen chloride. After the completion of the dropwise addition, the reaction was stirred at 25-30 C for 3 hours, and the basic reaction of the raw material III was monitored. After centrifugation, the filter cake was washed with purified water and dried to obtain pantoprazole sulfide wet product IV (solid). The wet product does not have to be dried and is directly fed to the next reaction. |
| 16.6 g |
With sodium hydroxide; In dichloromethane; at 20 - 30℃; for 2h; |
(1) 10 g of 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (Compound 1), 10 g of <strong>[97963-62-7]5-difluoromethoxy-2-mercapto-1H-benzimidazole</strong> (Compound 2), 100 ml of dichloromethane were added to the reaction flask. Stir. Add 130 g of sodium hydroxide solution with a concentration of 10%. Stir at 20-30 C for 2 h. standing layering, washing twice with dichloromethane, 30 ml each time, Then distilled under reduced pressure to give 16.6 g of yellow oil (Intermediate 3); |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping