|
With triethylamine; In dichloromethane; at 20℃; for 1h; |
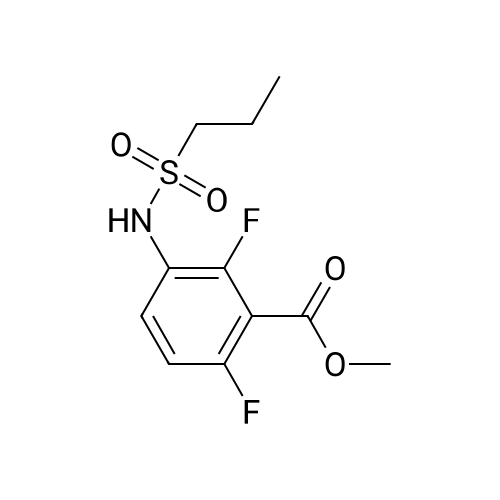
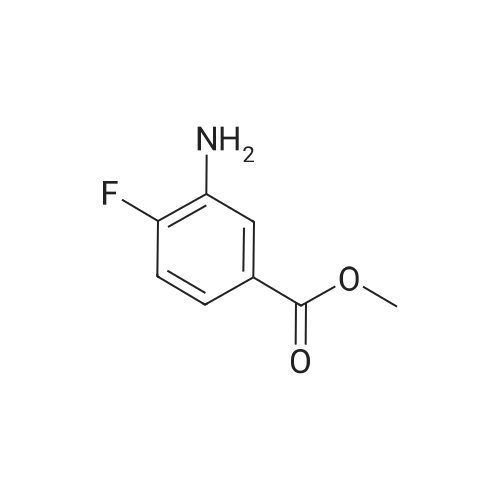
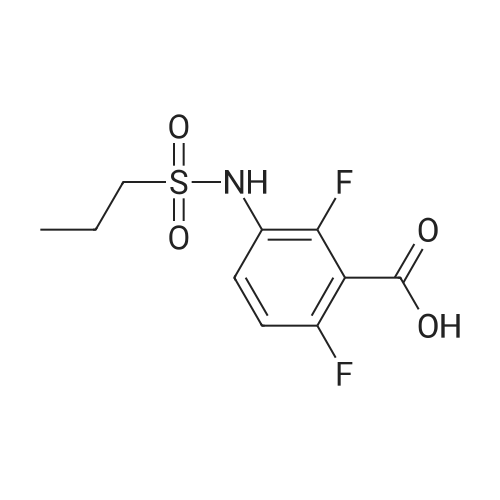
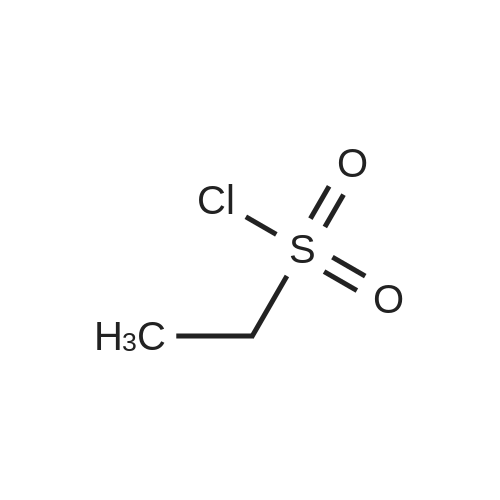
Propane- 1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL, 0.5 M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added, and the organic layer was separated, washed with water (2 x 300 mL), brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate ("EtOAc")/hexane. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(7V-(propylsulfonyl)propyl- Dulfonamide)benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, IH), 7.08-7.02 (m, IH), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J= 7.4 Hz, 6H). m/z (APCI-neg) M-(SO2Pr) = 292.2. |
|
With triethylamine; In dichloromethane; at 20℃; for 1h; |
Propane- 1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL, 0.5 M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added, and the organic layer was separated, washed with water (2 x 300 mL), brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate ("EtOAc")/hexane. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(iV-(propylsulfonyl)propyl- sulfonamido)benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, IH), 7.08-7.02 (m, IH), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02- 1.89 (m, 4H), 1.10 (t, J= 7.4 Hz, 6H). m/z (APCI-neg) M-(SO2Pr) = 292.2. |
|
With triethylamine; In dichloromethane; at 20℃; for 1h;Cool water bath; |
Step C: Propane- 1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL, 0.5M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added and the organic layer was separated, washed with water (2 X 300 mL) and brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate ("EtOAc")/hexane. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(7V-(propylsulfonyl)propylsulfonamido)benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, IH), 7.08-7.02 (m, IH), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J = 7.4 Hz, 6H). m/z (APCI-neg) M-(SO2Pr) = 292.2. |
|
With triethylamine; In dichloromethane; at 20℃; for 1h;Cooling with water bath; |
[00177] Step C: Propane- 1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL, 0.5M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added and the organic layer was separated, washed with water (2 X 300 mL) and brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate ("EtOAc")/hexane. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(N-(propylsulfonyl)propylsulfonamido)benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, IH), 7.08-7.02 (m, IH), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J = 7.4 Hz, 6H). m/z (APCI-neg) M-(SO2Pr) = 292.2. |
|
With triethylamine; In dichloromethane; at 20℃;Inert atmosphere; Cooling; |
Propane- 1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2C12 (175 mL, 0.5M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added and the organic layer was separated, washed with water (2 X 300 mL) and brine (200 mL), then dried (Na2S04), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate ("EtOAc")/hexane. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(N- (propylsulfonyl)propylsulfonamido)benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDC13) δ 7.52-7.45 (m, 1H), 7.08-7.02 (m, 1H), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J = 7.4 Hz, 6H). m/z (APCI-neg) M- (S02Pr) = 292.2. |
|
With triethylamine; In dichloromethane; at 20℃; for 3.5h;Cooling with ice; |
Methyl 2,6-difluoro-3-aminobenzoate compound 2310 (3.7 g, 0.020 mol) was dissolved in dichloromethaneTriethylamine (8.4 mL, 3.0 equiv) was added (40 mL), and propanesulfonyl chloride (4.7 mL, 2.1 mL) was added slowly under ice-cooling.Amount), stirring at room temperature for 3.5 hours, TLC test showed complete reaction. The reaction solution was quenched with water and extracted with dichloromethane for 3 times.Phase, 1M hydrochloric acid, saturated aqueous sodium bicarbonate solution, washed with water, saturated brine, dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressureThe oily compound 2311a was used in the next step without further purification. |
|
With triethylamine; In dichloromethane; at 20℃;Cooling with water bath; |
Propane- 1-sulfonyl chloride (23.46 niL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL, 0.5M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added and the organic layer was separated, washed with water (2 X 300 mL) and brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate/hexanes. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(N-(propylsulfonyl) propylsulfonamido)benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, IH), 7.08-7.02 (m, IH), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J = 7.4 Hz, 6H). m/z (APCI-neg) M-(SO2Pr) = 292.2. |
|
With triethylamine; In dichloromethane; at 20℃;Cooling; |
Step C: propane- 1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL, 0.5M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added and the organic layer was separated, washed with water (2 X 300 mL) and brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate/hexanes. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(N- (propylsulfonyl)propylsulfonamido)-benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, IH), 7.08-7.02 (m, IH), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J = 7.4 Hz, 6H). m/z (APCI-neg) M- (SO2Pr) = 292.2. |
|
With triethylamine; In dichloromethane; at 20℃;Cooling with water bath; |
Propane- 1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL, 0.5M) maintained in a cool water bath. The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added and the organic layer was separated, washed with water (2 X 300 mL) and brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% ethyl acetate ("EtOAc")/hexane. The isolated fractions were triturated with hexanes to afford methyl 2,6-difluoro-3-(N- (propylsulfonyl)propylsulfonamido)benzoate as a solid (24.4 g, 73% yield for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, IH), 7.08-7.02 (m, IH), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J = 7.4 Hz, 6H). m/z (APCI-neg) M- (SO2Pr) = 292.2. |
| 24.4 g |
With triethylamine; In dichloromethane; at 20℃; for 1h; |
Propane-1-sulfonyl chloride (23.46 mL, 209.3 mmol) was slowly added to a cooled solution of <strong>[84832-02-0]methyl 3-amino-2,6-difluorobenzoate</strong> (15.66 g, 83.7 mmol) and triethylamine (35.00 mL, 251.1 mmol) in CH2Cl2 (175 mL). The reaction mixture was stirred for 1 hour at room temperature. Water (300 mL) was added and the organic layer was separated, washed with water (2*300 mL) and brine (200 mL), then dried (Na2SO4), filtered and concentrated to an oil. The crude product was purified by column chromatography, eluting with 15% EtOAc/hexanes to afford methyl 2,6-difluoro-3-(N-(propylsulfonyl)propylsulfonamido)benzoate as a solid (24.4 g, 73% for 3 steps). 1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, 1H), 7.08-7.02 (m, 1H), 3.97 (s, 3H), 3.68-3.59 (m, 2H), 3.53-3.45 (m, 2H), 2.02-1.89 (m, 4H), 1.10 (t, J=7.4 Hz, 6H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +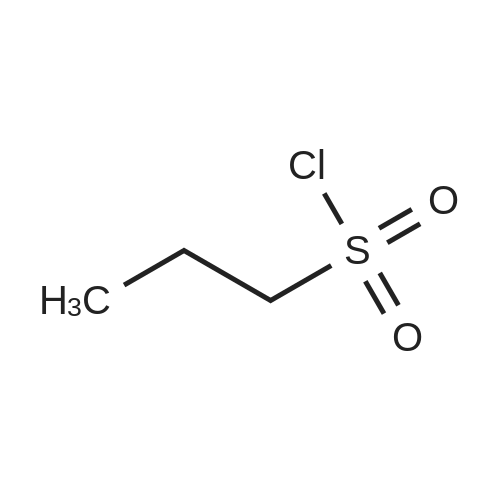

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping