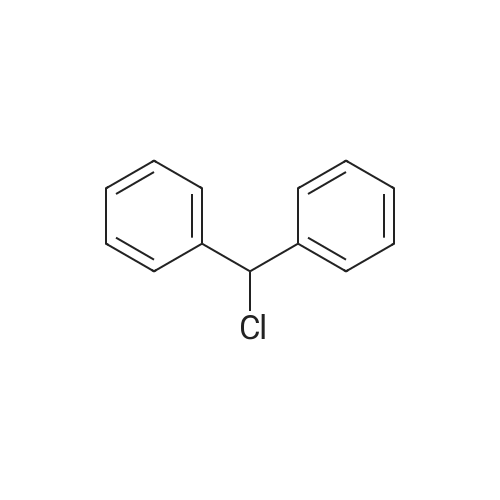
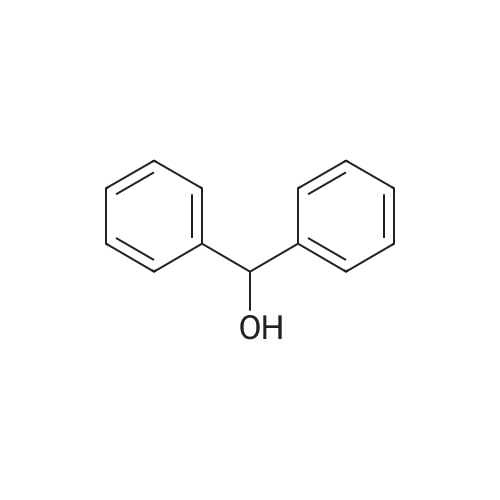
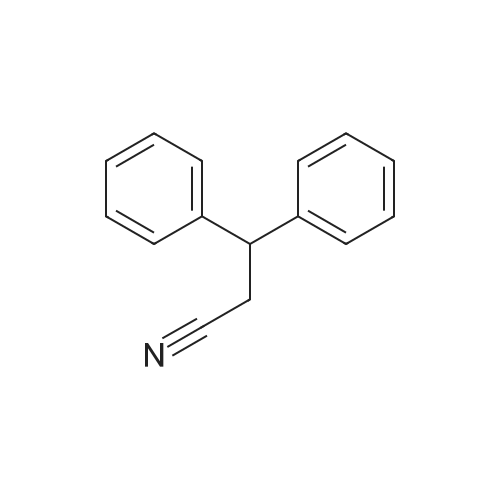
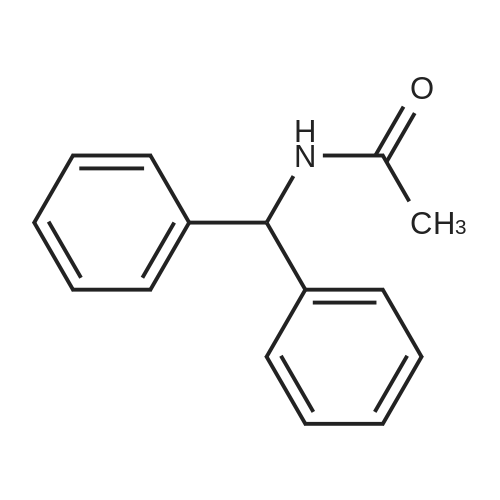
| 80% |
With n-butyllithium; In tetrahydrofuran; at -20℃; for 3h;Inert atmosphere; |
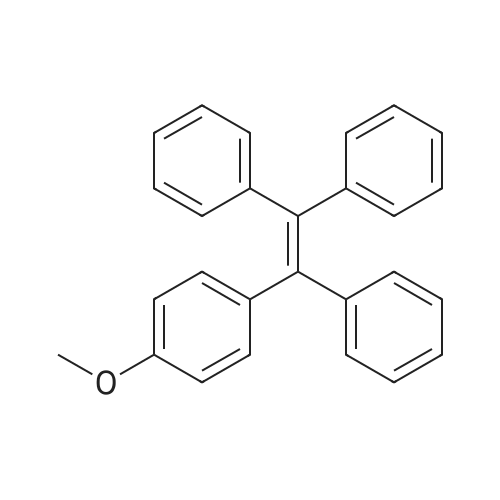
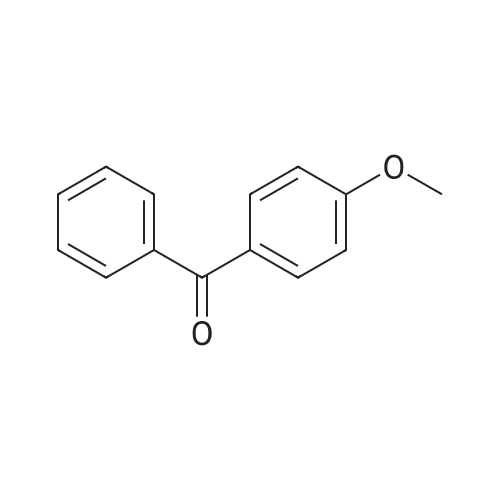
Diphenylmethane (12 mmol, 2.02 g)Dissolved in 50ml of tetrahydrofuran, minus 20 ,Under nitrogen, 2.5 M n-butyllithium (10 mmol, 4 ml) was added dropwise,4-Methoxybenzophenone (12 mmol, 3.03 g)Warmed to room temperature, stirred for 3 hours,Quenched with water, extracted with methylene chloride,Unscrew the solvent, add toluene,P-toluenesulfonic acid (1.8 mmol, 0.342 g),Reflux for 6 hours,Cool at room temperature, washed with 5% sodium bicarbonate twice, anhydrous magnesium sulfate dare to spin out the solvent to give a yellow crude product,Recrystallization gave white solid product Compound A, 80%. |
| 74% |
|
The diphenylmethane 4’ (3 g, 18 mmol) and the THF (20 mL) were added into the 100 mL flask. Replacing the gas in the flask 3 times under argon atmosphere, and the n-BuLi (7.5 mL, 18 mmol) was added at 0 oC dropwise, and the solution was stirred for 1 h. After that, the mixture was added into the 4-methoxylbenzophenone 5’ (3.4 g, 16 mmol) solution of THF (30 mL) at 0 oC. The solution was stirred at 30 oC for 6 h after 15 min. The mixture was poured into the solution of NH4Cl and was extracted with EA. The organic layer was washed with NaCl saturated aqueous solution, and dried with anhydrous sodium sulfate. The residue was evaporated and was added into the p-toluene sulfonic acid (0.69 g, 4 mmol) solution of toluene (120 mL). The solution was stirred at 120 oC for 6 h. The mixture was washed with saturated NaCl aqueous solution, dried, concentrated and purified by column chromatography on silica gel (SiO2, PE : DCM = 10:1, V/V) to give white solid (4.29 g, 74.0%). 1H NMR (400 MHz, CDCl3, ppm): δ = 7.10 - 6.98 (m, 15 H), 6.93 (d, J = 8.8 Hz, 2 H), 6.60 (d, J = 8.8 Hz, 2 H), 3.65 (s, 3 H). |
| 5.93 g |
|
(1) Under a nitrogen atmosphere,In SchlenkTube added dibenzyl burn(3.16 g, 20 mmol) and 80 mL of dry tetrahydrofuran. 2.2 M n-butyllithium in hexane (9. lmL, 20 mmo 1) was added dropwise at 0 C and the reaction was carried out at 0 C for 0.5 h.4-methoxybenzoylbenzene (3.40 g, 16 mmol) was added and the temperature was allowed to warm to room temperature and stirring was continued for 6 hours. After completion of the reaction, the reaction was quenched by the addition of saturated aqueous ammonium chloride solution, extracted with dichloromethane, the organic phase was collected, dried over anhydrous Na2S04,The solvent was evaporated to give the crude product as an intermediate. The intermediate was dissolved in 80 mL of dry toluene in a 250 mL round bottom flask,A catalytic amount of hydrated p-toluenesulfonic acid was added(570 mg, 3.0 mmol) and refluxed for 12 hours. After completion of the reaction, the mixture was cooled to room temperature, and the toluene solution was washed with 10 wt% aqueous NaHC03 solution. The organic phase was collected, the organic phase was collected, dried over anhydrous Na2S04, and the product was chromatographed on silica gel using petroleum ether as eluant. And the residue was dried in vacuo to give a white solid (5.93 g, yield 90.1%). The structure was characterized by Guru NMR. It was confirmed that the white solid was Compound 1, |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping