Inhibiting NOXO1 and CYBA binding to reduce NADPH oxidase I dependent ROS damage in skin explants
Senevirathne, Prasadini
;
Sterling, Alyssa
;
Anne Refaei, Mary
, et al.
Results Chem.,2023,6,101213.
DOI:
10.1016/j.rechem.2023.101213
More
Abstract: NADPH oxidases (NOXs) are newly identified enzymes that generate intracellular reactive oxygen species (ROS) in skin cells. Recent studies demonstrated that NOX1 holoenzyme is expressed in human keratinocytes and melanocytes, which are implicated in skin photo-carcinogenesis due to the high amounts of ROS produced. Holoenzyme activation requires a ternary complex comprised of NOX1, cytochrome B alpha chain (CYBA), and cytoplasmic NADPH Oxidase Organizer 1 (NOXO1) to properly form. By inhibiting this assembly process, an opportunity for reducing the production of catalytic ROS is possible, especially during high ROS conditions that occur under prolonged UV exposure. We designed a series of small mols. and evaluated their inhibitory effects on NOXO2 using in-silico docking methods in the 1WLP crystal structure. We show that the NOX_inh_5 inhibitor was successful in a variety of experiments using primary skin models from various skin tones. NOX_inh_5 proved to be non-cytotoxic while also improving the viability of primary human skin primary cells under UV exposure. Biophys. studies with NOX_inh_5 using an Isothermal calorimetric (ITC) binding and heteronuclear single quantum coherence (HSQC-NMR) exhibited inhibition of complex formation between NOXO2 and CYBA. Authentic human skin explants, treated with and without NOX_inh_5 and UV exposure, decreased p53 stabilization and decreased UV-induced DNA damage as quantified through cyclobutane dimer formation.
Keywords:
Reactive oxygen species ;
Apocynin ;
UV ;
Melanoma ;
Sunscreen ;
NOXO1 ;
CYBA
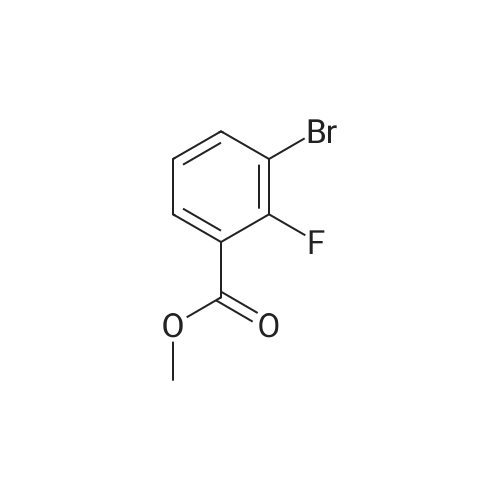
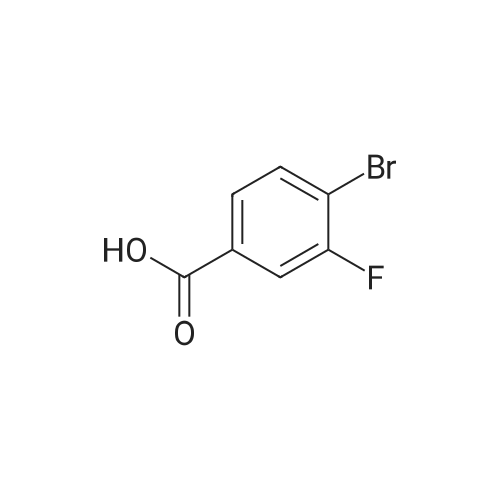
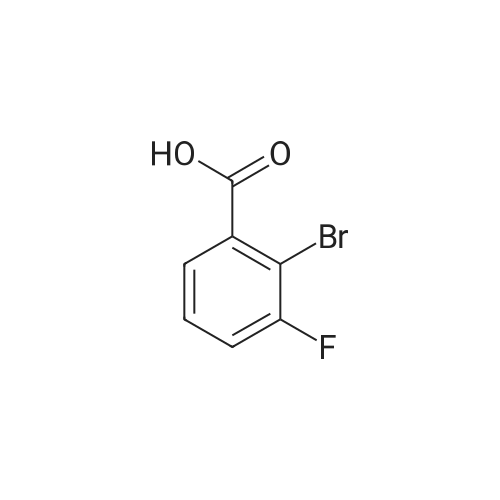
Purchased from AmBeed:
1007-16-5 ;
111-24-0 ;
583-55-1 ;
455-85-6
Development of reactive oxygen species (ROS) inhibitors and prodrugs for multiple applications
Senevirathne, Priyangika Prasadini
;
University of Cincinnati,2022.
More
Abstract: Reactive oxygen species are a group of highly reactive oxygen-containing entities that are important at a cellular level for multiple biological processes. Low concentrations of ROS can be beneficial as powerful signaling molecules in those biological processes, although excessive concentrations can promote high levels of DNA damage and a variety of diseases such as skin cancer. A newly identified intracellular ROS production source in skin cells is NADPH oxidases. Out of the NOX enzyme family, the NOX1 holoenzyme is most abundantly expressed in the human keratinocyte cells. UV radiation can trigger the activation of NOX1 isoforms which stimulate the assembling of member CYBA and the cytoplasmic protein NOXO1. Inhibition of these enzymes represents a catalytic approach toward reducing ROS for the prevention of ROS inducible diseases. Key disease states include melanoma induced by UV exposure. The first half of the dissertation focuses on investigating new small molecule inhibitors of a key NOX1 holoenzyme to address these challenges. We designed a series of molecules by optimizing the structure of diapocynin and evaluated by in-silico docking methods to determine the binding affinity with NOXO1 cytoplasmic protein (1WLP crystal structure). And have synthesized the series of target molecules for the structure-activity relationship studies. In the first section of the project, we discovered that inhibitor NOX_inh_5 was not cytotoxic, but instead improved the viability of human primary cells from UV exposure, decreased the cellular stress in human skin through the p53 pathway, and reduced the UV-induced DNA damage as monitored by quantification of cyclobutane dimer formation after UV exposure. Then, we characterized the inhibition potential of NOX_inh_5 by using an Isothermal calorimetric (ITC) binding assay and heteronuclear single quantum coherence (HSQC) technique and revealed that the candidate iii molecule can prevent the complex formation of NOXO1 and CYBA membrane protein. In the second section of the project, we did a structure-activity relationship study for the NOX_inh_5 small molecule to optimize the biological characteristics. The last section of the dissertation discussed the development of ROS sensible prodrug to combat the opioid overdose crisis. Here we used oxidative stress conditions caused by opioid overdose to activate the prodrug. Even though opioid antagonist naloxone has a high affinity to bind with opioid receptors to block opioid-induced activation, it is metabolically unstable and has a short half-life of around 33 min. We developed a peroxide-induced prodrug to overcome this issue that can release a steady stream of naloxone. This allows the concentration of naloxone to remain high for longer periods.
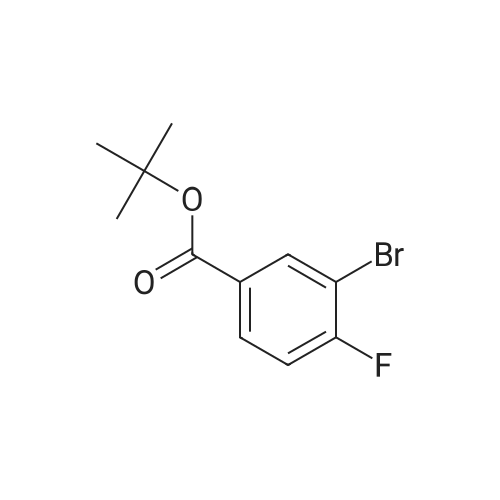
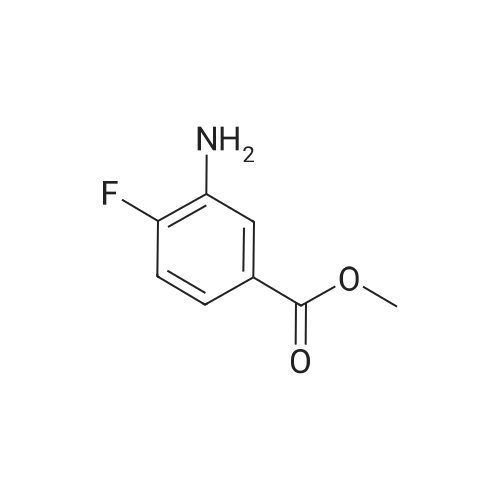
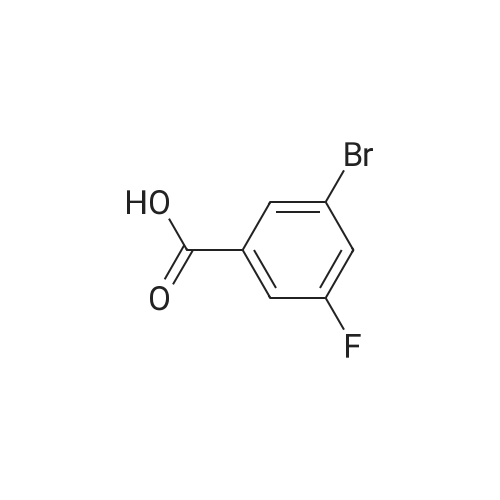
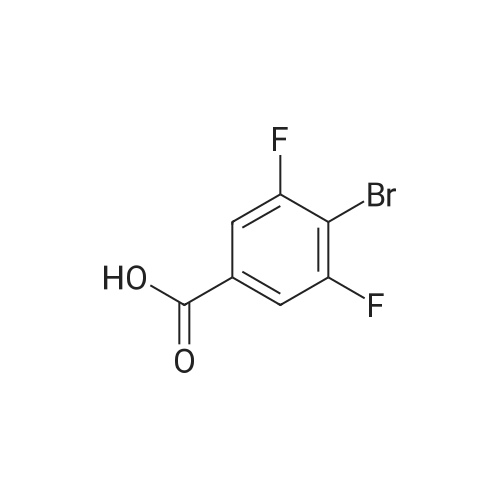
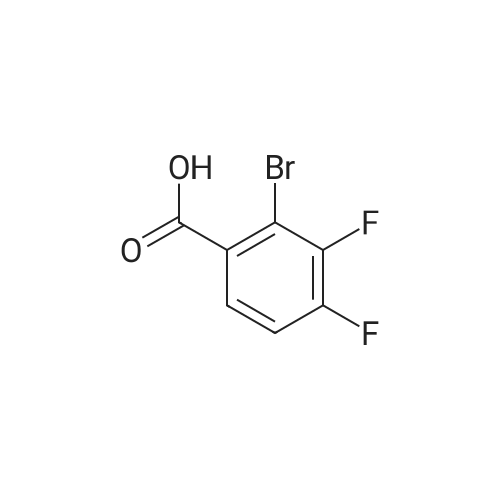
Purchased from AmBeed:
1007-16-5 ;
111-24-0 ;
14221-01-3 ;
99769-19-4 ;
351422-73-6 ;
158407-04-6 ;
1462-37-9 ;
583-61-9 ;
13965-03-2 ;
455-85-6 ;
148893-10-1

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping