| 97% |
With sodium carbonate;tetrakis(triphenylphosphine) palladium(0); In ethanol; water; toluene; at 80℃; for 20h; |
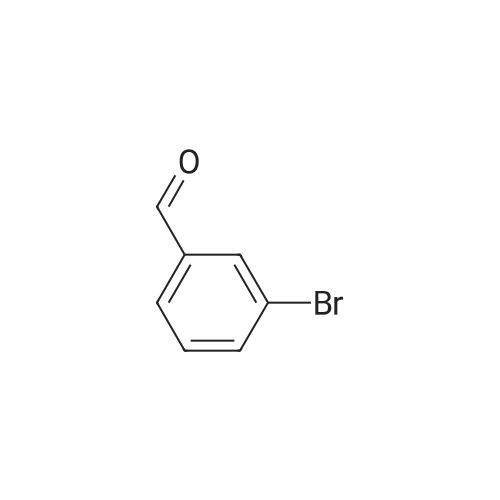
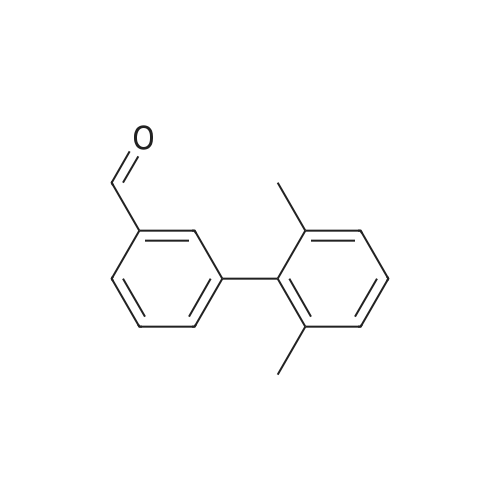
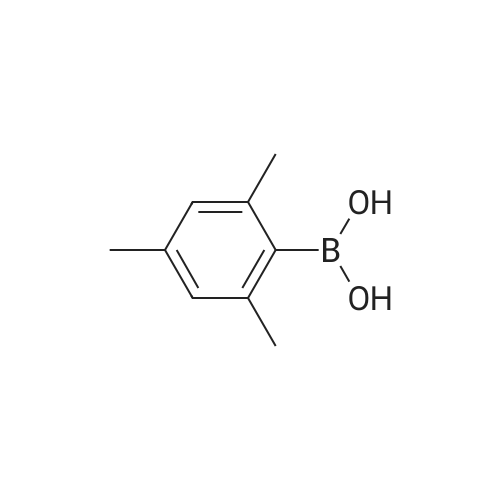
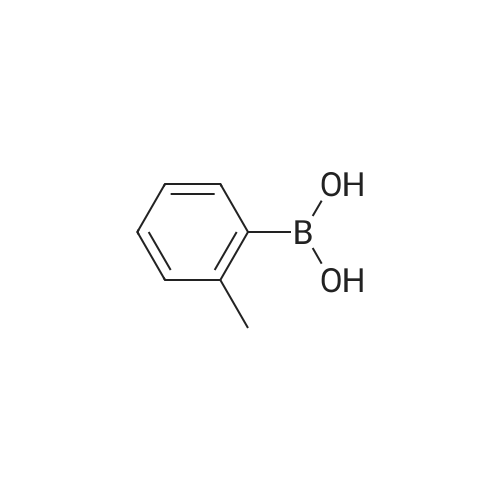
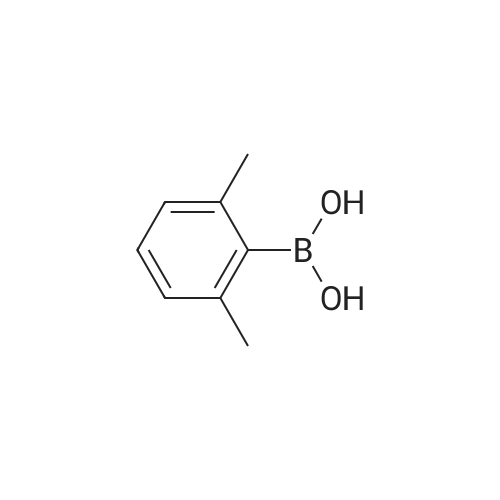
3-Bromobenzaldehyde (18.5 g, 100 mmol) and 2,6-dimethylphenylboronic acid (21.0 g, 140 mmol) were dissolved in a mixture of 1 M aqueous sodium carbonate solution (200 mL), ethanol (100 mL) and toluene (200 mL). The air was substituted with argon gas, and tetrakis(triphenylphosphine)palladium(0) (5.78 g, 5.00 mmol) was added. The reaction mixture was stirred under an argon atmosphere at 80C for 20 hr. After cooling the reaction mixture, water was added and the mixture was diluted with ethyl acetate. The insoluble material was filtered off through celite. The organic layer in the filtrate was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane-10% ethyl acetate/hexane) to give the title compound (20.4 g, yield 97%) as a colorless oil. MS m/z 211 (MH+). |
| 96% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In ethanol; water; toluene; at 80℃;Inert atmosphere; |
3-Bromobenzaldehyde (1.8 g, 10 mmol) and (2,6-dimethylphenyl)boronic acid (2.1 g, 14 mmol) were dissolved in a mixture of 1 M aqueous sodium carbonate solution (20 mL), EtOH (10 mL), and toluene (20 mL). After argon substitution, tetrakis(triphenylphosphine)palladium(0) (0.6 g, 0.5 mmol) was added. The reaction mixture was stirred under argon atmosphere at 80 C for 20 h. The reaction mixture was cooled, and water was added to the reaction mixture. The mixture was diluted with AcOEt, and the insoluble material was filtered through Celite. The organic layer of the filtrate was washed with brine, dried over anhydrous magnesium sulfate, and concentrated. The crude residue was purified by silica gel chromatograph using a mixture of petroleum ether/ethyl acetate (20 : 1, v/v) as eluent to afford the product 7a (2.1 g, 96%) as a colorless oil. 1H NMR (300 MHz, DMSO-d6) δ: 10.06 (s, 1H), 7.86-7.90 (m, 1H), 7.67-7.69 (m, 1H), 7.61 (t, J = 7.6 Hz, 1H), 7.42-7.46 (m, 1H), 7.11-7.23 (m, 3H), 2.02 (s, 6H). |
| 83% |
With tetrakis(triphenylphosphine) palladium(0); sodium hydrogencarbonate; In ethanol; toluene; at 80℃; for 22h;Inert atmosphere; |
3-bromo-benzaldehyde (370.4 mg, 2.002 mmol), 2,6- dimethyl phenyl boronic acid (420.5 mg, 2.804 mmol), saturated aqueous sodium hydrogen carbonate solution (1M solution, 4 mL, 4.001 mmol) ethanol (2.2 mL) of and in a mixed solution of toluene (4 mL), tetrakis (triphenylphosphine) palladium (0) (114.9 mg, 0.099 mmol) was added, under an argon atmosphere and stirred for 22 hours at 80 C.. The reaction mixture was cooled, water (5 mL) and ethyl acetate (10 mL) was added and filtered over Celite (registered trademark). Was separated into aqueous and organic layers, the aqueous layer was extracted twice with ethyl acetate (20 mL), the combined organic layers were extracted with brine (30 mL), further sedge aqueous layer of ethyl acetate (20 and extracted with mL). The combined organic layers were dried over sodium sulfate, the solvent was evaporated, and the residue was purified by column chromatography to give the title compound (hexane: diethyl ether = 60:: 1 ~ 50 1) (347.8 mg, 1.655 mmol, 83 %, to give a colorless oil). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping