| 59% |
In methanol; water; ethyl acetate; |
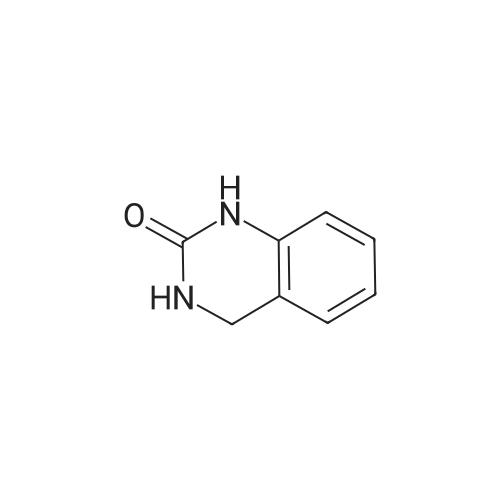
Example 3 3-(2,4-dioxo-1,4-dihydro-2H-quinazolin-3-yl)propionaldehyde Benzoylene urea (4.0 g, 24.7 mmol), Triton B (40 wt % in methanol) (11.0 mL, 24.7 mmol), water (80 mL) and methanol (400 mL) were combined at ambient temperature and stirred vigorously for 15 minutes. (until all the solids had gone into solution). To this colorless solution, acrolein (1.7 mL, 24.7 mmol) in methanol (20 mL) was added dropwise over 5 minutes. to give a yellow solution. The reaction was then heated to 55 C and stirred for 2 hours. and then at room temperature for approximately 16 hours. The yellow solution was concentrated to give a yellow oil which was taken up in ethyl acetate (25 mL) and water (50 mL). The aqueous layer was extracted again with ethyl acetate (25 mL). The organic layers were combined, washed with 1N HCl (20 mL), water (20 mL), saturated sodium bicarbonate solution (20 mL) and brine (20 mL), the organic layer was dried over magnesium sulfate and concentrated to give 3-[2,4-dioxo-1,4-dihydro-2H-quinazolin-3-yl]propionaldehyde as a yellow foam (3.2 g, 59%) which was used without further purification. The NMR data showed a purity of ~70%. NMR CDCl3 delta 9.85 (s, 1H), 8.10-8.06 (m, 1H), 7.63-7.57 (m, 1H), 7.24-7.19 (m, 1H), 7.13-7.07 (m, 1H), 4.44-4.40 (m, 2H), 2.85 (dt, 2H, J1,2=2 Hz, J1,3=7 Hz); MS=219 (p+1). |
| 59% |
In hydrogenchloride; methanol; water; ethyl acetate; |
Example 5 Preparation of 3-[2,4-Dioxo-1,4-dihydro-2H-quinazolin-3-yl]propionaldehyde Benzoylene urea (4.0 g, 24.7 mmol), Triton B (40 wt % in methanol) (11.0 mL, 24.7 mmol), water (80 mL) and methanol (400 mL) were combined at ambient temperature and stirred vigorously for 15 minutes. (until all the solids had gone into solution). To this colorless solution, acrolein (1.7 mL, 24.7 mmol) in methanol (20 mL) was added dropwise over 5 minutes. to give a yellow solution. The reaction was then heated to 55 C. and stirred for 2 hours. and then at room temperature for approximately 16 hours. The yellow solution was concentrated to give a yellow oil which was taken up in ethyl acetate (25 mL) and water (50 mL). The aqueous layer was extracted again with ethyl acetate (25 mL). The organic layers were combined, washed with IN HCl (20 mL), water (20 mL), saturated sodium bicarbonate solution (20 mL) and brine (20 mL), the organic layer was dried over magnesium sulfate and concentrated to give 3-[2,4-dioxo-1,4-dihydro-2H-quinazolin-3-yl]propionaldehyde as a yellow foam (3.2 g, 59%) which was used without further purification. The NMR data showed a purity of 70%. NMR CDCl3 delta 9.85 (s, 1H), 8.10-8.06 (m, 1H), 7.63-7.57 (m, 1H), 7.24-7.19 (m, 1H), 7.13-7.07 (m, 1H), 4.44-4.40 (m, 2H), 2.85 (dt, 2H, J1,2=2 Hz, J1,3=7 Hz); MS=219 (p+1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping