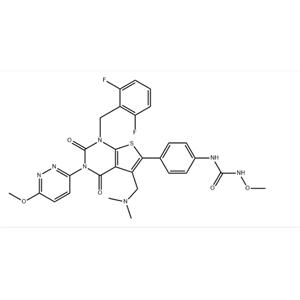
Relugolix NEW
| Price | Get Latest Price | ||
| Package | 1kg | 10kg | 100kg |
| Min. Order: | 1kg |
| Supply Ability: | 20tons |
| Update Time: | 2023-08-04 |
Product Details
| Product Name: Relugolix | CAS No.: 737789-87-6 |
| Min. Order: 1kg | Purity: 99% |
| Supply Ability: 20tons | Release date: 2023/08/04 |
Elutinib property Melting point 153-158 ° C Boiling point 715.0 ± 60.0 ° C (Predicted) Density 1.34 Storage condition -20 ° C Solubility SolubleinDMSO (uptoatleast25mg/ml) Acid dissociation constant (pKa) 4.09 ± 0.30 (Predicted) Morphology Solid color Whiteoroff white stability Stablefor1yearfromdateofpurchaseasupplied.SolutionsinDMSOmayrestoredat-20 ° Cforupto3months.InChIKeyXYFPWWZEPKGCCK-GOSISDBHSA-NSMI LESC (N1CCC [C @ @ H] (N2C3C (C (C4=CC=C (OC5=CC=CC=C5)) C=C4)=N2)=C (N) N=CN=3) C1) (=O) C=CCAS database 936563-96-1 Usage and synthesis method of Irutinib Irutinib is a Bruton Tyrosine kinase (BTK) inhibitor, which is used to treat Chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Both MCL and CLL belong to B-cell non Hodgkin lymphoma, which is difficult to cure and easy to relapse. The commonly used chemoimmunotherapy is not targeted, and grade 3 or 4 adverse reactions often occur. Irutinib can target BTK, which is essential for the formation, differentiation, information transmission, and survival of B lymphocytes, and irreversibly inhibit the activity of BTK, effectively inhibiting the proliferation and survival of tumor cells; After oral administration, the absorption is rapid, reaching the maximum blood drug concentration within 1-2 hours. The adverse reactions belong to level 1 or 2, and will become a new choice for the treatment of CLL and MCL. On November 13, 2013, the United States Food and Drug Administration (FDA) reported a melting point of 228 ° C (decomp) (Solv: ethylacetate (141-78-6)) for the Ruilugoli property; Tetrahydrofuran (109-99-9) density 1.442 ± 0.06g/cm3 (Predicted) storage conditions Storeat-20 ° C solubility DMSO: 2.0 (MaxConc. mg/mL); 32.1 (MaxConc. mM) Ethanol: 1.0 (MaxConc. mg/mL); 1.6 (MaxConc. mM) Form Acrystallinesolid Acid dissociation constant (pKa) 13.17 ± 0.70 (Predicted) InChIKeyAOMXMOCNKJTRQP-UHFFFAOYSA-NSMILESN (C1=CC=C (C2SC3=C (C=2CN (C) C) C (=O) N (C2=NN=C (OC) C=C2) C (=O) N3CC2=C (F) C=CC=C2F) C=C1) C (NOC)=O Introduction to the use and synthesis method of Ruilugoli Li has been evaluated among nearly 1600 study participants in Phase 1, Phase 2 and Phase 3 clinical trials. Relugolix can rapidly reduce the estrogen and Progestogen levels of women when taken orally once a day. Takeda Pharmaceutical compared the safety and effectiveness of Relugolix and Leupreelin in the treatment of uterine fibrosis with menorrhagia, as well as the safety and effectiveness of the above two drugs in the treatment of pain symptoms related to uterine fibrosis through a series of clinical phase III studies conducted in Japan, and finally confirmed the safety and effectiveness of relagolix in the treatment of uterine Fibroma. In addition, Takeda Pharmaceuticals conducted a phase II clinical study on relugolix for endometriosis and prostate cancer, confirming that relugolix can significantly reduce pain caused by endometriosis, reduce serum testosterone to castration levels, and significantly reduce prostate specific antigen (PSA). Apply Relugolix, with the chemical name of N - (4- (1- (2,6-difluorobenzyl) -5- ((dimethylamino) methyl) -3- (6-neneneba methoxy -3-pyridazinyl) -2,4-dioxo-1,2,3,4- Tetrahydrothiophene o [2,3-d] pyrimidin-6-yl) phenyl) - N '- Methoxy group urea. It is a new drug jointly developed by Myovant Company and Takeda Pharmaceutical Co., Ltd. It is a small molecule Gonadotropin releasing hormone (GnRH) receptor antagonist and has potential for use in indications such as Fibroma of uterus, endometriosis and prostate cancer.
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $31.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-13 | |
| $0.00/1KG |
VIP1Y
|
Hangzhou Hyper Chemicals Limited
|
2024-09-06 | |
| $0.00/10mg |
VIP1Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-08-13 | |
| $0.10/1KG |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2024-08-05 | |
| $0.00/1g |
VIP1Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-07-24 | |
| $5.00/25kg |
Ouhuang Engineering Materials (Hubei) Co., Ltd
|
2024-04-25 | ||
| $1.00/1g |
VIP4Y
|
Dorne Chemical Technology co. LTD
|
2024-03-26 | |
| $0.00/1kg |
VIP1Y
|
Shandong Hanjiang Chemical Co., Ltd
|
2024-01-22 | |
| $0.00/1g |
VIP1Y
|
Biopole Pharmatech Co., Ltd.
|
2024-01-19 | |
| $0.00/25KG |
VIP5Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-08-14 |



 China
China