
Liraglutide NEW
| Price | Get Latest Price |
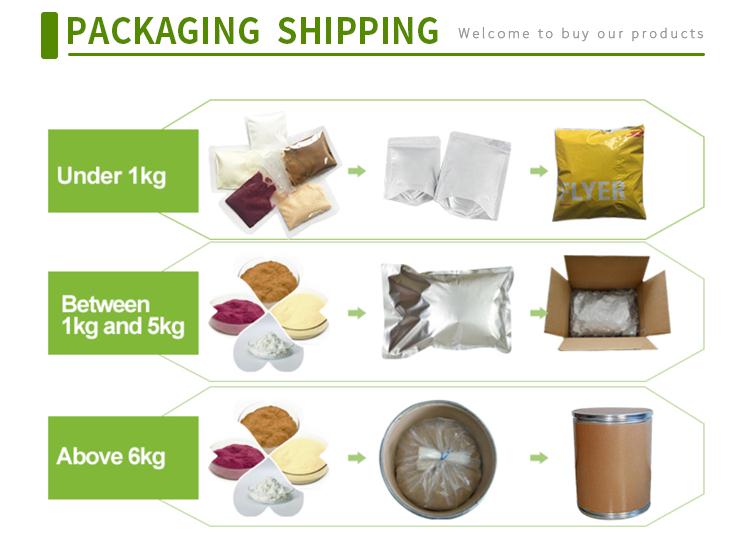
| Package | 25kg |
| Min. Order: | 1kg |
| Supply Ability: | 2000ton |
| Update Time: | 2024-04-12 |
Product Details
| Product Name: Liraglutide | CAS No.: 204656-20-2 |
| EC-No.: 810-818-7 | Min. Order: 1kg |
| Purity: 99% | Supply Ability: 2000ton |
| Release date: 2024/04/12 | |
| Appearance: White Powder |
Liraglutide is a clear, colorless solution. The dosage form is subcutaneous injection. After more than 10 years of research and development, Liraglutide has many effects such as promoting islet cell regeneration, lowering blood sugar, reducing weight and protecting the cardiovascular system. The brand name Victoza is used to treat type 2 diabetes, and the brand name Saxenda is used to treat chronic obesity as a supplement for diet control and physical exercise. Liraglutide is an acylated human glucagon-like peptide-1 (GLP-1) receptor agonist, which has 97% amino acid sequence homology with human endogenous GLP-1 (7-37), retains all the biological activity of GLP-1, and has GLP-1 receptor agonist effect. The molecular structure of GLP-1 differs from that of natural GLP-1 by only one amino acid, and GLP-1 is modified in two parts: lysine 34th is replaced by arginine, and a glutamate-mediated 16-carbon palmitoyl fatty acid side chain is added to lysine 26th.
Drug interaction
Liraglutide causes a delay in the time to empty the stomach, which may affect the absorption of oral medications taken simultaneously. In clinical pharmacological trials, liraglutide did not affect the oral drug absorption tested at a clinically relevant level. Nonetheless, monitor the potential consequences of delayed absorption of oral medications taken with liraglutide.
Pharmacological action
1 Protective effect on β-cells of pancreatic islets
2 The effect of reducing body mass
3 Cardiovascular effects of liraglutide
4 Protective effect on liver
5 Therapeutic effects on Alzheimer's disease (AD)
Adverse reaction
The incidence of hypoglycemia during liraglutide treatment was very low. Gastrointestinal discomfort is the most common adverse effect of liraglutide treatment, and slowly increasing the dosage of liraglutide can reduce the proportion of dose-related nausea. Gastrointestinal discomfort was most common during the first week of liraglutide treatment, with diarrhea and nausea occurring most frequently, most of which were transient, mild, or tolerable and related to the dose of liraglutide.
1. Gastrointestinal reactions During the treatment of liraglutide, the most common adverse reaction is gastrointestinal discomfort, with nausea and diarrhea being more common, which are transient reactions and can be tolerated. At the dose of 1.8mg daily, the average incidence of gastrointestinal discomfort was 25%, with the highest incidence in the first 4 weeks and decreasing over time.
2. Immunogenicity A case of skin rash and blistering after the use of Liraglutide injection was reported, which is extremely rare in clinical practice, but the adverse reaction may also be caused by some substances in the prescription. Less than 10% of patients in the LEAD study tested positive for liraglutide antibodies, and the side effect did not affect the effect of lowering blood sugar.
3. Medullary thyroid cancer In rodent data, Liraglutide has been found to cause thyroid C-cell hyperplasia, increasing the risk of C-cell tumors. Thyroid C cell hyperplasia is considered to be the precursor of medullary thyroid carcinoma. Studies have shown that liraglutide at doses similar to those used in humans caused benign tumors of thyroid C cells in rats and mice, and 8 times the highest dose used in humans caused C-cell malignancies in rats and mice. The relevance of these findings to medullary thyroid cancer in humans is unclear.
4. Acute pancreatitis In a non-clinical trial of 1.0mg?kg-1?d-1 subcutaneously administered to ZDF rats for 13 weeks, lilaplutide did not increase biochemical and histopathological risk markers of pancreatitis, and pancreatic exocrine was not affected. However, clinical studies of pancreatic histopathological changes in primates are still necessary for human risk assessment. The FDA's Center for Adverse Event Reporting reported 7 cases of pancreatitis among 4257 patients treated with lilaglutide in Phase II and Phase III clinical trials. But people with type 2 diabetes are three times more likely to develop pancreatitis themselves, and it is difficult to determine whether the complication is related to the medication.
Storage Condition | Keep in a cool and dry place |
Transportation | By Sea or by Air(DHL/UPS/TNT/FEDEX/EMS) |
Delivery Time | 7-28 days |
Payment | T/T, Western Union or Bitcoin |









Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1Box |
VIP1Y
|
American HealthyMorph LLC
|
2024-12-26 | |
| $100.00/1box |
VIP4Y
|
Hebei Lingding Biotechnology Co., Ltd.
|
2024-12-25 | |
| $119.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-15 | |
| $0.00/1BOX |
VIP1Y
|
Shandong Huizhihan Supply Chain Co., Ltd
|
2024-11-12 | |
| $500.00/1gram |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-08 | |
| $0.00/1box |
VIP5Y
|
Jinan Jianfeng Chemical Co., Ltd
|
2024-08-07 | |
| $0.10/1KG |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2024-07-25 | |
| $50.00/1kg |
VIP1Y
|
Shandong Deshang Chemical Co., Ltd.
|
2024-07-10 | |
| $0.00/10g/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2024-06-27 | |
| $1.00/1g |
VIP4Y
|
Dorne Chemical Technology co. LTD
|
2024-06-25 |
- Since: 2020-08-05
- Address: 804, Unit 2, Building 4, i City, No. 11, South Tangyan Road, High-tech Zone, Xi 'an, Shaanxi











 China
China