Lead(II) carbonate basic NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-21 |
Product Details
| Product Name: Lead(II) carbonate basic | CAS No.: 1319-46-6 |
| EC-No.: 215-290-6 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/21 |
| CAS: | 1319-46-6 |
| MF: | 2CO3.2Pb.H2O2Pb |
| MW: | 775.63 |
| EINECS: | 215-290-6 |
| Product Categories: | Lead Salts;Metal and Ceramic Science;Salts;Analytical Reagents;Analytical Reagents for General Use;Puriss;Inorganics |
| Mol File: | 1319-46-6.mol |
 | |
| Lead(II) carbonate basic Chemical Properties |
| Melting point | 400 °C (dec.)(lit.) |
| density | 6,14 g/cm3 |
| form | Powder |
| color | White to off-white |
| Specific Gravity | 6.14 |
| Water Solubility | Insoluble |
| Solubility Product Constant (Ksp) | pKsp: 13.13 |
| Exposure limits | ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 1319-46-6(CAS DataBase Reference) |
| EPA Substance Registry System | Basic lead carbonate (1319-46-6) |
| Lead(II) carbonate basic Usage And Synthesis |
| Solubility in water(g/100ml) | Dissolved grams per 100 milliliters of water: 7.269 × 10-5/20 °C Toxicity |
| Chemical properties | White powder, hexagonal crystal system. It is insoluble in water and ethanol, and soluble in acetic acid and nitric acid. Gamma-Butyrolactone |
| Uses | Lead carbonate basic has a high refractive index and weather fastness, widely used in pigments, coatings, plastics, printing and dyeing, analysis reagents, etc .; for pigments, it is used for excellent pearlescent pigments; and it is also used to produce inorganic pigments with paint and pigments. In the printing and ink, it is used for packaging paper, business cards, plastic cloth, textiles and so on. As the possibility of leading to lead poisoning in the production and application, and the vulnerability to thicken when the paint is made by white lead, and the whiteness easily declining when contact with and hydrogen sulfide and other shortcomings, its application is limited. While the coating film produced by the lead white is more stable, with excellent weather resistance and rust resistance. In the paint industry, it is still used as the white pigments for the production of the original paint, anti-rust paint and outdoor paint; raw materials for ceramic glaze, painting and cosmetic; amino or acrylic baking finish of coating. Add 2% to 8%. It is used in painting with cars, motorcycles, bicycles, furniture and so on. In plastic, basic lead carbonate can be used as vinyl chloride plastic stabilizer for the production of pearlescent plastic, pearlescent paint and so on. |
| Preparation | 1. Chemical method: adding carbon dioxide, deionized water into reaction solution prepared by lead acetate, lead oxide and deionized water. After reaction, then by precipitation, added with nitrocellulose pulp slurry, precipitation, crystallization, centrifugal dehydration, washed with alcohol, drying, the basic lead carbonate is obtained. Alcohol waste liquid is treated and recovered. The reaction equation is: Pb(Ac)2+PbO+H2O→Pb(Ac)2?Pb(OH)2; 3[Pb(Ac)2?Pb(OH)2]+2CO2→3Pb(Ac)2+2PbCO3?Pb(OH)2+2H2O 2. Acetic acid method: mix yellow lead, acetic acid and water together in the mother liquor tank, and the concentration of the mixture of lead oxide is 230~250 g/L. Suspend the mixture with stirring; keep warm for 3h in the 90 ℃ leading to the formation of basic lead acetate; clarify and carbonize with purified carbon dioxide; when 85% lead hydroxide in the basic lead acetate is carbonized, that is the end of the reaction; and then by precipitation, centrifugal separation, with oil and alcohol washing and drying, basic lead carbonate products is made. In addition, the separated mother liquor can be recycled. The reaction equation is: 2PbO + 2HAc → Pb (Ac) 2 ? Pb (OH) 2 3[Pb(Ac)2?Pb(OH)2]+2CO2→3Pb(Ac)2+2PbCO3?Pb(OH)2+2H2O. |
| Toxicity | Early symptoms of poisoning is that lead linear appearing in the edge of the gums and urinary poisoning. For chronic poisoning, it has changes in the nervous system, the emergence of debilitating syndrome, encephalopathy, dysmotility, changes in the blood system, metabolic and endocrine disorders, gastrointestinal changes and changes in the cardiovascular system. A maximum allowable concentration of lead and lead inorganic compounds is 0.01 mg/m3 and 0.0007 mg/m3 in an average working day. When the poisoned suffered colic, he should receive subcutaneous injection of atropine, morphine, and intravenous injection of magnesium sulfate, sodium thiosulfate, calcium chloride. Masks blocking 95% to 97% of lead dust is allowable when working; when in the environment of high vapor concentration, you can use the filter gas mask for a forced supply of fresh air. When the poisoned ones suffered colic, he should receive subcutaneous injection of atropine, morphine, and intravenous injection of magnesium sulfate, sodium thiosulfate, calcium chloride. |
| Chemical Properties | Basic lead carbonate forms white hexagonal crystals; it decomposes when heated to 400 °C. It is insoluble in water and alcohol, slightly soluble in carbonated water, and soluble in nitric acid. |
| Chemical Properties | dense white powder |
| Uses | Lead(II) carbonate basic can be used as pigment in oil paints and water colors; in cements; for making putty and lead carbonate paper; in the processing of parchment. |
| Uses | Lead(II) carbonate is used in ceramic glaze and exterior paint. |
| Definition | Acid-soluble, heavy, white powder or crystalline substance, insoluble in water and alco- hol. |
| Preparation | Many commercial processes have been developed for manufacturing basic lead carbonate. These include: Thomson-Stewart process, Carter process, and Dutch process. The method of preparation involves treating lead with acetic acid vapors in the presence of carbon dioxide at 60°C. In the Thomson-Stewart process, finely divided lead monoxide or lead metal is mixed with water to give aqueous slurry, which is then mixed with acetic acid in the presence of air and carbon dioxide. All these processes are slow, taking weeks to obtain products of desired composition. Basic lead carbonate also is precipitated by dissolving lead monoxide in lead(II) acetate solution, and treating the solution with carbon dioxide. It also is produced by electrolysis of sodium nitrate or sodium acetate using lead anode and then precipitating out the product by adding sodium carbonate. . |
| Production Methods | Basic Lead Carbonate is produced by several methods, in which soluble lead acetate is treated with carbon dioxide. For example, in the Thompson-Stewart process, an aqueous slurry of finely divided lead metal or monoxide, or a mixture of both, is treated with acetic acid in the presence of air and carbon dioxide. High quality, very fine particle-size basic lead carbonate is produced, ranging in carbonate content from 62 to 65% (vs 68.9% PbCO3, theoretical). |
| Flammability and Explosibility | Non flammable |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
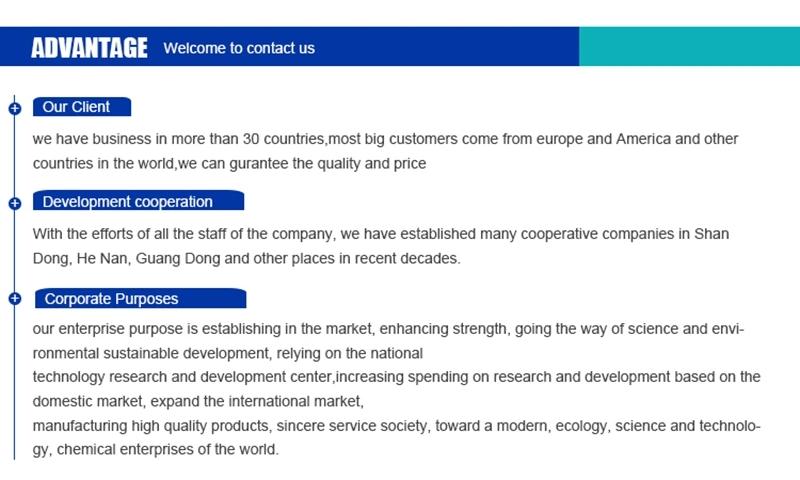
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $45.00/1kg |
VIP1Y
|
Hebei Zhuanglai Chemical Trading Co Ltd
|
2024-12-24 | |
| $0.00/1KG |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2024-09-04 | |
| $100.00/1KG |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-21 | |
| $0.00/25kg |
Shaanxi Haibo Biotechnology Co., Ltd
|
2023-09-01 | ||
| $88.00/1KG |
Wuhan Dujiang Industrial Co., Ltd.
|
2021-11-12 | ||
| $1.00/1KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2021-02-20 | |
| $3.00/1KG |
VIP7Y
|
Career Henan Chemical Co
|
2019-07-04 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 Japan
Japan