Isavuconazole NEW
| Price | Get Latest Price |
| Package | 1KG |
| Min. Order: | 1KG |
| Supply Ability: | 1000KGS |
| Update Time: | 2024-08-15 |
Product Details
| Product Name: Isavuconazole | CAS No.: 241479-67-4 |
| Min. Order: 1KG | Purity: 98% |
| Supply Ability: 1000KGS | Release date: 2024/08/15 |
Email: sales@anbuchem.com
Whatsapp:+8618638608485
24 hours online, Welcom to inquiry.
Isavuconazole Basic information
Product Name: Isavuconazole
Synonyms: Isaconazole;4-[2-[(2R,3R)-3-(2,5-difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)butan-2-yl]-1,3-thiazol-4-yl]benzonitrile;Isavucozole;4-(2-((2R,3R)-3-(2,5-difluorophenyl)-3-hydroxy-4-(1H-1,2,4-triazol-1-yl)butan-2-yl)thiazol-4-yl)benzonitrile;Isavuconazonium;Isavuconazole(BAL-4815;Isavuconazole;4-[2-[(1R,2R)-2-(2,5-Difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]-4-thiazolyl]benzonitrile
CAS: 241479-67-4
MF: C22H17F2N5OS
MW: 437.47
EINECS: 1592732-453-0
Product Categories: Inhibitors;Aromatics;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;Isavuconazonium sulfate;API
Mol File: 241479-67-4.mol
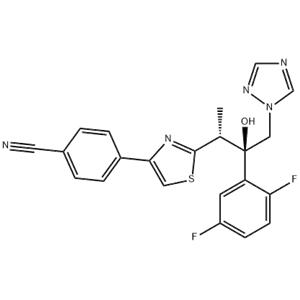
Isavuconazole Structure
Isavuconazole Chemical Properties
Melting point 89 - 91oC
Boiling point 678.0±65.0 °C(Predicted)
density 1.38
storage temp. 2-8°C
solubility Chloroform (Slightly), Methanol (Slightly)
form Solid
pka 11.42±0.29(Predicted)
color Off-White to Beige
InChI InChI=1S/C22H17F2N5OS/c1-14(21-28-20(10-31-21)16-4-2-15(9-25)3-5-16)22(30,11-29-13-26-12-27-29)18-8-17(23)6-7-19(18)24/h2-8,10,12-14,30H,11H2,1H3/t14-,22+/m0/s1
InChIKey DDFOUSQFMYRUQK-RCDICMHDSA-N
SMILES C(#N)C1=CC=C(C2=CSC([C@H](C)[C@](C3=CC(F)=CC=C3F)(O)CN3C=NC=N3)=N2)C=C1
Safety Information
MSDS Information
Isavuconazole Usage And Synthesis
Description Isavuconazonium, the water-soluble pro-drug of isavuconazole, is developed by Basilea Pharmaceutica. Isavuconazole, like other azoles, inhibits ergosterol synthesis and is a water-soluble compound with both oral and intravenous formulations. It has excellent bioavailability and a long elimination half-life, allowing for once-weekly dosing. It is the only broad-spectrum water-soluble triazole available.
Uses Isavuconazole is a new triazole currently undergoing phase III clinical trials. This compound has shown in vitro activity against a large number of clinical important yeasts and molds including Aspergillus spp., Fusarium spp., Scedosporium spp., Candida spp., the Zygomycetes and Cryptococcus spp.
Definition ChEBI: Isavuconazole is a 1,3-thiazole that is butan-2-ol which is substituted at positions 1, 2, and 3 by 1,2,4-triazol-1-yl, 2,5-difluorophenyl, and 4-(p-cyanophenyl)-1,3-thiazol-2-yl groups, respectively. It is an antifungal drug used for the treatment of invasive aspergillosis and invasive mucormycosis. It has a role as an ergosterol biosynthesis inhibitor, an EC 1.14.13.70 (sterol 14alpha-demethylase) inhibitor and an orphan drug. It is a member of 1,3-thiazoles, a nitrile, a difluorobenzene, a tertiary alcohol, a triazole antifungal drug and a conazole antifungal drug.
Antimicrobial activity Isavuconazole is a new broad-spectrum triazole with activity against Candida, Cryptococcus, Aspergillus, Zygomycetes, Rhizopus, and Rhizomucor spp. and dimorphic fungi including Histoplasma capsulatum and Blastomyces dermatitidis and a range of dermatophytes. In animal models, isavuconazole is highly effective against systemic candidiasis and disseminated Aspergillus fumigatus and flavus infections.
Biochem/physiol Actions Maximum plasma concentrations of BAL 4815 are observed 1.5–3 hours after oral doses or at the end of the 1-hour intravenous infusion. Mean elimination half-lives are remarkably long more than 50 and 70 hours after oral and intravenous doses, respectively, allowing for once-daily (or less frequent) dosing. The volume of distribution is large and systemic clearance low. The protein binding is 98%. Escalation in multiple dosing regimens results in a proportional increase in maximum plasma drug concentration.
Clinical Use Three regimens of isavuconazonium (BAL 8557) – single 200 mg dose followed by 50 mg daily, single 400 mg followed by 100 mg daily and single 400 mg followed by 400 mg weekly –were demonstrated to be as efficacious as fluconazole in a phase II study conducted on patients with uncomplicated esophageal candidiasis. The results of a phase II open-label dose-escalating trial of intravenous isavuconazonium as prophylaxis in AML (NCT00413439) are pending (NIH, 2008). Two phase III randomized double-blind studies to evaluate the safety and efficacy of isavuconazole are currently under way, versus caspofungin followed by voriconazole in the treatment of candidaemia and invasive candidiasis and versus voriconazole in primary treatment of invasive filamentous fungal infection . A phase III open-label study using isavuconazole in patients with aspergillosis and renal impairment and those with rare fungi is being planned NCT00634049.
Toxicology In animals, isavuconazole has revealed no mutagenic, allergenic, phototoxic, or irritant potential. In 15 healthy volunteers, single doses of isavuconazole (up to 200 mg) were well tolerated, and no severe or serious adverse events occurred. In a multiple-dose pharmacokinetic study, 24 healthy males received isavuconazole for 21 days of oral or 14 days of intravenous dosing. The most frequent adverse events were headache, nasopharyngitis, and rhinitis, and all were mild or moderate; however one subject on oral high-dose BAL 8557 had mild transient abnormal liver function on day 14 of therapy, which resolved despite continuing the drug. No clinically relevant changes in vital signs or electrocardiogram were observed. In a double-blind randomized phase II trial for the treatment of esophageal candidiasis, BAL 8557 was safe and well tolerated, with an adverse event profile similar to that of fluconazole, the comparator drug.
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $31.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $10.00/1kg |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-15 | |
| $30.00/1Kg |
VIP3Y
|
Xinxiang Hongqi District Houyuan Trading Co.,Ltd
|
2023-05-31 | |
| $0.00/25Kg/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2023-05-11 | |
| $200.00/1kg |
Hebei Mingeng Biotechnology Co., Ltd
|
2022-11-28 | ||
| $0.00/1g/Bag |
VIP4Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2021-07-13 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $1.00/1KG |
VIP7Y
|
Career Henan Chemical Co
|
2018-08-20 | |
| $0.00/25Kg/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2023-05-11 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-09 |
- Since: 2019-12-09
- Address: No 10 Yuying Road, Guancheng District, Zhengzhou 450000, China
+86-15988602810
sales@anbuchem.com




 China
China