
Denosumab NEW
| Price | Get Latest Price |
| Package | 25kg |
| Min. Order: | 1kg |
| Supply Ability: | 2000ton |
| Update Time: | 2024-04-12 |
Product Details
| Product Name: Denosumab | CAS No.: 615258-40-7 |
| Min. Order: 1kg | Purity: 99% |
| Supply Ability: 2000ton | Release date: 2024/04/12 |
| Appearance: Colourless Liquid |
Pharmacological action:
Dinomumab is the first approved monoclonal antibody specifically targeting a RANK ligand. RANK ligand is a transmembrane or soluble protein necessary for osteoclasts to maintain their structure, function and survival. The mRNA of human RANKL is mainly found in bone, bone marrow and lymphoid tissue, and its main role in bone is to stimulate the differentiation and activity of osteoclasts and inhibit the apoptosis of osteoclasts. Osteoclasts are responsible for bone reabsorption, and the precursors of osteoclasts must have low levels of macrophage colony-stimulating factor and RANKL in the process of differentiation into mature osteoclasts. Dinomumab has a high affinity with RANKL, which prevents RANK ligand from activating RANK on the surface of osteoclasts, inhibits osteoclast activation and development, reduces bone resorption, increases bone density and bone strength of cortical bone and bone trabecular bone, promotes bone reconstruction, and reduces the incidence of vertebral, non-vertebral and hip fractures in postmenopausal women with osteoporosis. The effect of dinomumab on bone remodeling can be evaluated by measuring some bone renewal markers, such as N-terminal peptide, a marker of bone resorption, and bone-specific alkaline phosphatase, a marker of bone formation.
Adverse reaction
The most common adverse events reported in clinical trials were back pain (34.7%), limb pain (11.7%), musculoskeletal pain (7.6%), hypercholesterolemia (7.2%), and cystitis (5.9%). The most common adverse events leading to drug withdrawal were breast cancer, back pain, and constipation. Other common adverse reactions include: Anemia (3.3%), angina (2.6%), atrial fibrillation (2.0%), dizziness (5.0%), abdominal pain (3.3%), flatulence (2.2%), gastroesophageal reflux disease (2.1%), peripheral edema (4.9%), weakness (2.3%), upper respiratory tract infection (4.9%), pneumonia (3.9%), pharyngitis (2.3% ), herpes zoster (2.0%) and osteoarthritis (2.1%) of the spine, sciatica (4.6%), insomnia (3.2%), rash (2.5%), itching (2.2%).
Storage Condition | Keep in a cool and dry place |
Transportation | By Sea or by Air(DHL/UPS/TNT/FEDEX/EMS) |
Delivery Time | 7-28 days |
Payment | T/T, Western Union or Bitcoin |





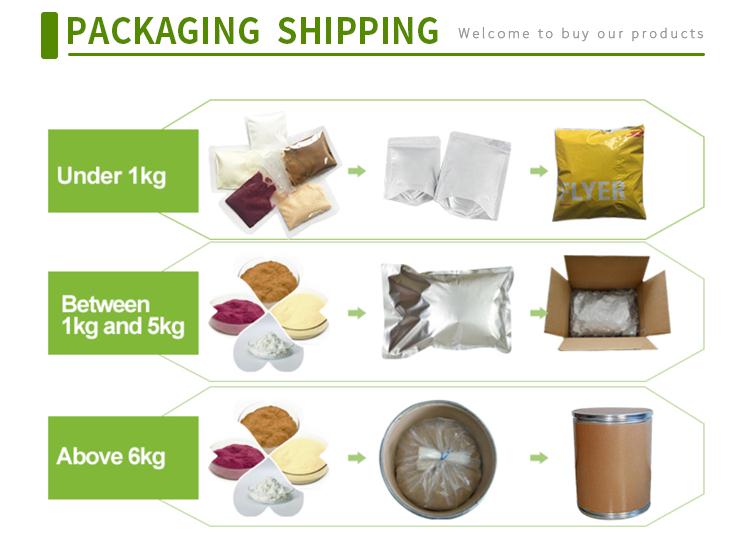




Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $775.00/10mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $0.00/1mg |
VIP3Y
|
Wuhan Senwayer Century Chemical Co.,Ltd
|
2023-02-09 | |
| $0.00/10mg |
Hangzhou Huarong Pharm Co., Ltd.
|
2021-09-15 | ||
| $1.10/1g |
VIP4Y
|
Dideu Industries Group Limited
|
2021-07-02 | |
| $0.00/1Kg |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-04-30 |
- Since: 2020-08-05
- Address: 804, Unit 2, Building 4, i City, No. 11, South Tangyan Road, High-tech Zone, Xi 'an, Shaanxi










 China
China