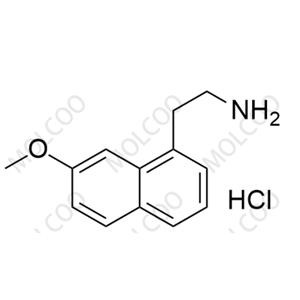
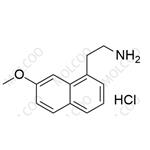
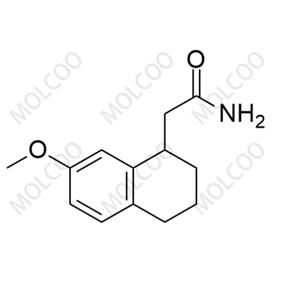
Agomelatine Impurity NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 1000000 |
| Update Time: | 2025-01-10 |
Product Details
| Product Name: Agomelatine Impurity | CAS No.: 139525-77-2 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 1000000 | Release date: 2025/01/10 |
We can provide a full range of impurity reference/standard products required for drug development. Most of the impurities are synthesized through the process, there are also many items of impurities can not be obtained by synthetic means, need to be obtained through raw materials, intermediates, crude products or side reactions contained in the trace target compounds, Hubei Moke has a professional impurity preparation and separation technology team, equipped with professional SFC preparation and separation equipment. It can carry out efficient and accurate separation of impurities for complex projects, and solve the problem of impurity preparation for customers.

Agomelatine Impurity Reference Standards
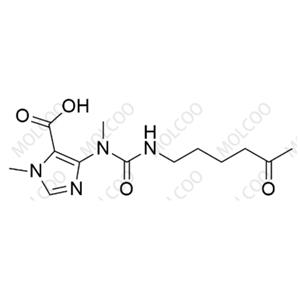
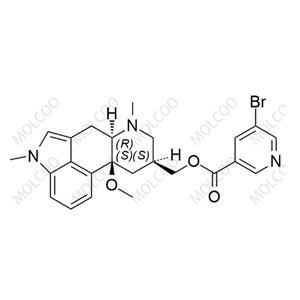
Agomelatine impurity reference standards serve as crucial reference materials for pharmaceutical testing and analysis, primarily used for calibrating instruments and equipment, establishing working standards, evaluating analytical methods, and ensuring quality assurance. The agomelatine impurity reference standards we provide encompass various potential impurity types, including but not limited to 7-methoxy-3,4-dihydronaphthalen-1(2H)-one, 2-(7-methoxynaphthalen-1-yl)acetic acid, 7-methoxy-1-naphthol, 2-(7-methoxynaphthalen-1-yl)acetonitrile, bis(2-(7-methoxynaphthalen-1-yl)ethyl)amine, and others. These impurity reference standards have undergone meticulous preparation and rigorous quality control to ensure their purity and structural accuracy.
We offer agomelatine impurity reference standards in various packaging specifications to meet the needs of different customers. Additionally, we provide customization services, allowing us to prepare specific impurity reference standards according to customers' specific requirements.
In the process of pharmaceutical research and development, production, and quality control, agomelatine impurity reference standards play a vital role. They help ensure the safety and effectiveness of pharmaceutical products, safeguarding patients' medication safety.
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $30.00/5mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $55.00/1kg |
VIP2Y
|
Zibo Hangyu Biotechnology Development Co., Ltd
|
2023-11-06 | |
| $7.00/1kg |
VIP7Y
|
Career Henan Chemical Co
|
2018-12-17 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China