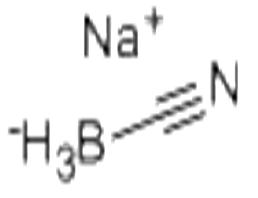
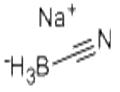
Sodium cyanoborohydride
| Price | $1 |
| Package | 1KG |
| Min. Order: | 1G |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Sodium cyanoborohydride | CAS No.: 25895-60-7 |
| Min. Order: 1G | Purity: 98% |
| Supply Ability: 100KG | Release date: 2019/07/06 |
AD68
| Sodium cyanoborohydride Basic information |
| Product Name: | Sodium cyanoborohydride |
| Synonyms: | (cyano-C)trihydro-,sodium,(T-4)-Borate(1-);sodium,(beta-4)-borate(1-(cyano-c)trihydro-;SODIUM CYANOBOROHYDRIDE;SODIUM CYANOTRIHYDRIDOBORATE;SODIUM CYANOTRIHYDROBORATE;Sodium cyonobrohydriole;SODIUM CYANOBOROHYDRIDE, 5.0M SOLUTION I N AQUEOUS CA. 1M SODIUM HYDROXIDE;SODIUM CYANOBOROHYDRIDE, 1.0M SOLUTION I N TETRAHYDROFURAN |
| CAS: | 25895-60-7 |
| MF: | CH3BNNa |
| MW: | 62.84 |
| EINECS: | 247-317-2 |
| Product Categories: | Selectivity reducing agent;B (Classes of Boron Compounds);Classes of Metal Compounds;Na (Sodium) Compounds (excluding simple sodium salts);Reduction;Synthetic Organic Chemistry;Tetrahydroborates;Typical Metal Compounds;Borohydrides;Synthetic Reagents;borane |
| Mol File: | 25895-60-7.mol |
 |
|
| Sodium cyanoborohydride Chemical Properties |
| Melting point | >242 °C (dec.)(lit.) |
| Boiling point | 307°C |
| density | 1.083 g/mL at 25 °C |
| Fp | −1 °F |
| storage temp. | Store under Argon |
| solubility | H2O: may be clear to slightly hazy |
| form | Powder |
| color | White |
| Water Solubility | 2120 g/L at 29 ºC (dec.) |
| Sensitive | Moisture Sensitive |
| Merck | 14,8606 |
| Stability: | Stable. Hygroscopic. Reacts violently with water, giving off and igniting hydrogen. Do not use water on fires involving this chemical - instead use dry soda ash. Incompatible with strong acids, water, strong oxidizing agents. |
| CAS DataBase Reference | 25895-60-7(CAS DataBase Reference) |
| EPA Substance Registry System | Borate(1-), (cyano-.kappa.C)trihydro-, sodium, (T-4)-(25895-60-7) |
| Safety Information |
| Hazard Codes | T+,N,T,F |
| Risk Statements | 26/27/28-32-34-50/53-16-15-11-51/53-36-23/24/25-19-14-40-36/37/38 |
| Safety Statements | 26-28-36/37/39-45-60-61-8-50A-43-28A-1-16-36/37 |
| RIDADR | UN 3179 4.1/PG 2 |
| WGK Germany | 1 |
| F | 10-21 |
| Hazard Note | Toxic/Highly Flammable |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | I |
| HS Code | 28500020 |
| Sodium cyanoborohydride Usage And Synthesis |
| Chemical Properties | White solid |
| Uses |
|
| Uses | Selective reducing agent for aldehydes, ketones, oximes, enamines; does not reduce amides, ethers, lactones, nitriles, nitro Compounds and epoxides. Also used for reductive amination of ketones and aldehydes, reductive alkylation of amines and hydrazines, reductive displacement of halides and tosylates, deoxygenation of aldehydes and ketones. See Lane, loc. cit. |
| Reducing Agents | Sodium cyanoborohydride are frequently used for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound. Contact with strong acids liberates the highly toxic gas HCN. A safer reducing agent with comparable reactivity is sodium triacetoxyborohydride. Reduction with Sodium Cyanoborohydride:
 |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1kg |
VIP1Y
|
Wuhan Circle Star Chem-medical Technology Co.,Ltd
|
2025-01-08 | |
| $10.00/1KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-10-30 | |
| $690.00/1Kg |
VIP1Y
|
R&D Scientific Inc.
|
2024-08-28 | |
| $200.00/1kg |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-20 | |
| $0.00/25kg |
VIP1Y
|
Hebei Mojin Biotechnology Co.,Ltd
|
2024-07-29 | |
| $75.00/1kg |
VIP1Y
|
Hebei Zhuanglai Chemical Trading Co.,Ltd
|
2024-06-07 | |
| $20.00/1kg |
VIP1Y
|
hebei hongtan Biotechnology Co., Ltd
|
2024-05-16 | |
| $0.00/1kg |
VIP1Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-29 | |
| $8.00/1kg |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2024-04-24 | |
| $20.00/1kg |
Shaanxi Franta Biotechnology Co., Ltd
|
2024-03-28 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
楊俊青
sales@coreychem.com
sales@coreychem.com



![1,4-BIS-[4-(6-ACRYLOYLOXYHEXYLOXY)BENZOYLOXY]-2-METHYLBENZENE](https://img.chemicalbook.com/ProductImageEN/2018-8/Large/c574aa67-91f2-48bc-8056-39f72c7708bb.gif)

 China
China