AST2818 mesylate manufacturers
- Alflutinib mesylate
-
- $71.00 / 1mg
-
2024-11-19
- CAS:2130958-55-1
- Min. Order:
- Purity: 99.81%
- Supply Ability: 10g
- AST2818 mesylate
-
- $0.00 / 1kg
-
2022-09-27
- CAS:2130958-55-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton
|
| | AST2818 mesylate Basic information |
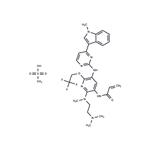
| Product Name: | AST2818 mesylate | | Synonyms: | N-[2-[[2-(Dimethylamino)ethyl]methylamino]-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]-6-(2,2,2-trifluoroethoxy)-3-pyridinyl]-2-propenamide compd. with methanesulfonate (1:1);AST2818 mesylate;Alflutinib mesylate (AST2818);Alflutinib methanesulfonate;Alflutinib (AST2818 mesylate);Alflutinib mesylate;Alflutinib mesylate (Furmonertinib);Furmonertinib mesylate | | CAS: | 2130958-55-1 | | MF: | C29H35F3N8O5S | | MW: | 664.71 | | EINECS: | | | Product Categories: | | | Mol File: | 2130958-55-1.mol | 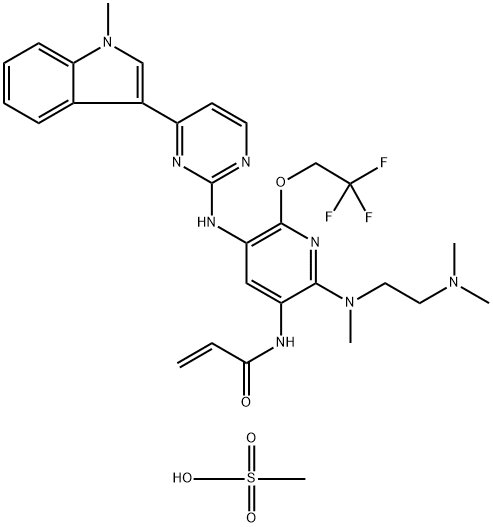 |
| | AST2818 mesylate Chemical Properties |
| storage temp. | Store at -20°C,unstable in solution, ready to use. | | solubility | Chloroform: 10 mg/ml; DMF: slightly soluble; DMSO: slightly soluble | | form | A crystalline solid | | color | Light yellow to yellow |
| | AST2818 mesylate Usage And Synthesis |
| Description |
Furmonertinib (formerly known as alflutinib) is another me-too third-generation EGFR TKI that has a pyridyl ring replacing the phenyl ring of osimertinib and a trifluoroethyl group replacing the methyl group (Fig. 4). This TKI, which was discovered and developed by Shanghai Allist Pharma, was approved in November 2019 by the NMPA of China for the second-line treatment of NSCLC with EGFR sensitive mutation and EGFR T790M drug-resistant mutation, and more recently as a first-line treatment for classical EGFR mutant NSCLC. In June 2022, furmonertinib was granted the Fast Track designation by the FDA in patients with advanced or metastatic NSCLC with activating EGFR or HER2 mutations, including exon 20 insertion mutations.
|
| | AST2818 mesylate Preparation Products And Raw materials |
|