Torezolid
- CAS No.
- 856866-72-3
- Chemical Name:
- Torezolid
- Synonyms
- Tedizolid;(R)-3-(3-fluoro-4-(6-(2-Methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-5-(hydroxyMethyl)oxazolidin-2-one;Tedizolidted;CS-1826;Tedizolid Impurity 47;DA-7157;DA 7157;Torezolid;Torezolid-21;Torezolid (TR-701
- CBNumber:
- CB02484972
- Molecular Formula:
- C17H15FN6O3
- Molecular Weight:
- 370.34
- MDL Number:
- MFCD19442562
- MOL File:
- 856866-72-3.mol
| Melting point | 201 °C |
|---|---|
| Boiling point | 614.5±65.0 °C(Predicted) |
| Density | 1.57 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Water (Slightly, Heated) |
| form | Solid |
| pka | 14.05±0.10(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C17H15FN6O3/c1-23-21-16(20-22-23)15-5-2-10(7-19-15)13-4-3-11(6-14(13)18)24-8-12(9-25)27-17(24)26/h2-7,12,25H,8-9H2,1H3/t12-/m1/s1 |
| InChIKey | XFALPSLJIHVRKE-GFCCVEGCSA-N |
| SMILES | O1[C@@H](CO)CN(C2=CC=C(C3=CC=C(C4=NN(C)N=N4)N=C3)C(F)=C2)C1=O |
| CAS DataBase Reference | 856866-72-3 |
| FDA UNII | 97HLQ82NGL |
| ATC code | J01XX11 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361-H373 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P260-P314-P501 |
| HS Code | 29339900 |
Torezolid price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | T013750 | Tedizolid | 856866-72-3 | 50mg | $1190 | 2021-12-16 | Buy |
| Matrix Scientific | 153201 | (R)-3-(3-Fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-5-(hydroxymethyl)oxazolidin-2-one 95% | 856866-72-3 | 1g | $1323 | 2021-12-16 | Buy |
| Matrix Scientific | 143684 | 3-[3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone 95% | 856866-72-3 | 250mg | $1832 | 2021-12-16 | Buy |
| Matrix Scientific | 143684 | 3-[3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone 95% | 856866-72-3 | 100mg | $916 | 2021-12-16 | Buy |
| ChemScene | CS-0687 | Tedizolid 99.46% | 856866-72-3 | 10mg | $216 | 2021-12-16 | Buy |
Torezolid Chemical Properties,Uses,Production
Novel antibacterial drugs
Tedizolid belongs to the second generation oxazolidinone antibiotics, being an analog of linezolid as well as a kind of protein synthesis inhibitors, acting on the bacterial ribosomal 50S subunit and causing inhibition of bacterial protein synthesis. Compared with linezolid, the efficacy of the two is basically the same, but linezolid required to be taken for 2 times a day and for 10 continuous days while Tedizolid only takes 1 time daily and continuous taking for 6 days.
On June 20, 2014, the US FDA had approved a new antimicrobial drug Tedizolid (trade name: Sivextro) for the treatment of skin infections in adult patients. Sivextro is approved for the treatment of acute bacterial skin and skin infections caused by certain sensitive bacteria such as Staphylococcus aureus (including methicillin-resistant strains and methicillin-sensitive strains), various streptococci and Enterococcus faecalis (ABSSSI). Sivextro is administered intravenously and orally.
Tedizolid phosphate is a second-generation oxazolidinone antibiotic developed by Dong-A Pharmaceutical, licensed to Cubist Pharmaceuticals and Bayer for commercial development.
Sivextro is designed for the treatment of serious or life-threatening infections, and its listing applications are eligible for Qualified Infectious Disease Products (QIDP) and have obtained the approve of FDA for accelerated review. Sivextro's QIDP eligibility gives the drug exclusive five-year market exclusivity, in addition to its market exclusivity under the Food, Drug, and Cosmetic Act.
The safety and efficacy of Sivextro were evaluated in two clinical trials involving 1315 ABSSSI adult patients. Subjects were randomized to Sivextro or another antibiotic linezolid approved for the treatment of acute bacterial skin and skin structure infections (ABSSSI). The results showed that its clinical efficacy is equivalent to linezolid. However, it will cause less gastrointestinal adverse reactions and thrombocytopenia adverse reactions than linezolid. The incidence of drug resistance is also lower. Tedizolid has been shown to be more tolerant than vancomycin.
The most common side effects identified in clinical trials include nausea, headache, diarrhea, vomiting and dizziness. The safety and efficacy of Sivextro have not been evaluated in patients with reduced white blood cell levels (neutropenia), so alternative therapies should be considered for these patients. Sivextro is marketed by Cubist Pharmaceuticals, Inc., based in Lexington, Massachusetts.
Status of intellectual property: Compound patent ZL200480037612.2, protection period 2024.12.17 expires.
This information was edited by Xiao Nan from Chemicalbook (2015-08-14).
Indications
Tedizolid phosphate is an oxazolidinone compound used in the treatment of acute bacterial skin and skin structure infections caused by the following gram-positive bacteria-sensitive strains: Staphylococcus aureus (including Methicillin-resistant and methicillin-susceptible strains), pyogenic streptococci, Streptococcus lactis, Streptococcus angustifolia (including Angina, Streptococcus intermedius and Streptococcus constellation), and Enterococcus faecalis.
State of Intellectual Property
(A) Administrative protection, new drug protection and new drug monitoring period
On August 9, 2013, Bayer Pharmaceuticals has filed a new drug application with CFDA.
(B) Domestic patent
1. Patented compounds
East Asia Pharmaceutical has applied for the compound patent in China under the application number is ZL200480037612.2. The date of application is December 17, 2004; the protection period will expire at 2024.12.17. Three subsequent divisional applications were further filed with the application numbers being 201010508824.1 (preparation), 201110304983.4, 201210155386.4.
On October 9, 2009, the Teresius Therapeutics Company has applied for a preparation method patent with the patent number of 200980140144.4.
2. Crystal patent
On February 3, 2010, Teulius Therapeutics applied for a crystalline form patent of free acid with the application number of 201080014363.0. Form I of the disclosed free acid has the advantage that it is more stable than the disodium salt and has no moisture absorption.
3. Formulation Composition Patents
The above-mentioned patent 200480037612.2 and its divisional application patent have disclosed the Tedizolid powder, tablet, capsule and injection.
The above-mentioned patent 201080014363.0 have disclosed the prescription of Tedizolid tablets and freeze-dried preparations.
Uses
Tedizolid, known as TR-700, is an oral and i.v administered intracellular antibacterial drug.
Definition
ChEBI: A member of the class of pyridines that is pyridine which is substituted by a 2-methyl-2H-tetrazol-5-yl group at position 2 and by a 2-fluoro-4-[(5R)-5-(hydroxymethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl group at position 5 It is used as its phosphate pro-drug used for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains (MRSA) and meth cillin-susceptible strains), various Streptococcus species, and Enterococcus faecalis.
Biological Activity
tedizolid is an oxazolidinone antimicrobial agent with mic50 value of 0.5 μg/ml for mssa, mrsa, vr e. faecium and vr e. faecalis and 0.25 μg/ml for msse, mrse, pssp and prsp [1].the resistant gram-positive infection is a serious global health problem. for instant, the methicillin-resistant s. aureus (mrsa) has spread all over the world with rates ranging from 18 to 26 cases among 100,000 people. besides that, there come out a serious of resistant strains such as the linezolid-resistant s. aureus (lrsa) and the vancomycin-resistant s. aureus (vrsa). due to the unfavorable outcomes of the existed antibiotics, alternative treatments have been developed. tedizolid is a synthetic antibiotic that works based on the inhibition of protein synthesis. it binds to the 50s ribosome and inhibits the formation of the 70s complex [1].tedizolid showed potent bacteriostatic activity against many resistant gram-positive pathogens such as mssa, mrsa, s. pyogenes and s. pneumoniae. for the enterococcal and staphylococcal isolates, tedizolid displayed more than 4-fold higher potency than that of linezolid. it also showed inhibitory effects on a panel of 169 linezolid-resistant staphylococcal isolates with 79.2% inhibition at concentration of ≤ 4μg/ml. the mic values of tedizolid against linezolid-resistant staphylococci were in a range from 0.06 to 16 mg/l. besides that, tedizolid was found to be the inhibitors of human monoamine oxidase with ic50 values of 8.7 and 5.7 μm for mao-a and mao-b, respectively [1, 2 and 3].when treated in vivo, tedizolid was the active moiety converted from the pro-drug tedizolid phosphate. it was found that granulocytes could affect the antistaphylococcal effect of tedizolid. in neutropenic mice, the administration of tedizolid for 24 hours or 48 hours caused ed50 values of 25.2 and 35.7 mg/kg/day, respectively [1 and 4].
References
[1] kanafani z a, corey g r. tedizolid (tr-701): a new oxazolidinone with enhanced potency. expert opinion on investigational drugs, 2012, 21(4): 515-522.
[2] rodríguez-avial i, culebras e, betriu c, et al. in vitro activity of tedizolid (tr-700) against linezolid-resistant staphylococci. journal of antimicrobial chemotherapy, 2012, 67(1): 167-169.
[3] flanagan s, bartizal k, minassian s l, et al. in vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. antimicrobial agents and chemotherapy, 2013, 57(7): 3060-3066.
[4] louie a, liu w, kulawy r, et al. in vivo pharmacodynamics of torezolid phosphate (tr-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant staphylococcus aureus strains in a mouse thigh infection model. antimicrobial agents and chemotherapy, 2011, 55(7): 3453-3460.
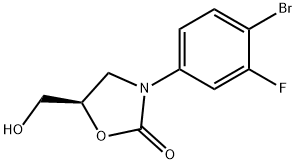
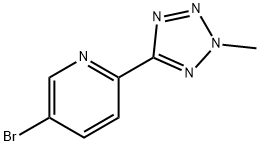

Torezolid Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12835 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| Cangzhou Kangrui Pharma Tech Co. Ltd., | +86-18632776803 +86-13833998158 | cangzhoukangrui@126.com | China | 738 | 58 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| NINGBO INNO PHARMCHEM CO., LTD. | 13867897135 | sales@nbinno.com | CHINA | 923 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | stella@zetchem.com | China | 2136 | 58 |
| Changzhou Ansciep Chemical Co., Ltd. | +86 519 86305871 | sales@ansciepchem.com | CHINA | 4241 | 58 |
Related articles
- Synthesis of Tedizolid
- Tedizolid is the first FDA-approved second-generation oxazolidinone antibiotic, which is clinically used to treat skin and ski....
- Aug 17,2022
View Lastest Price from Torezolid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
![3-[3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone pictures](/ProductImageEN1/2024-11/Small/6ab9084a-df0f-43c9-8549-85328a4152f1.gif) |
2024-12-19 | 3-[3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone
856866-72-3
|
US $0.00-0.00 / g | 100g | 99 | 20kgs | Cangzhou Kangrui Pharma Tech Co. Ltd., | |
 |
2024-11-21 | Torezolid
856866-72-3
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-11-01 | Torezolid
856866-72-3
|
US $0.00-0.00 / KG | 1KG | 99% | 500KG | Hangzhou Hyper Chemicals Limited |
-
![3-[3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone pictures](/ProductImageEN1/2024-11/Small/6ab9084a-df0f-43c9-8549-85328a4152f1.gif)
- 3-[3-Fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone
856866-72-3
- US $0.00-0.00 / g
- 99
- Cangzhou Kangrui Pharma Tech Co. Ltd.,
-

- Torezolid
856866-72-3
- US $10.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Torezolid
856866-72-3
- US $0.00-0.00 / KG
- 99%
- Hangzhou Hyper Chemicals Limited
856866-72-3(Torezolid)Related Search:
1of4





