85666-17-7
中文名稱
U-63557A
英文名稱
U-63557A
CAS
85666-17-7
分子式
C15H10NNaO3
分子量
275.23
MOL 文件
85666-17-7.mol
更新日期
2024/11/07 22:28:47
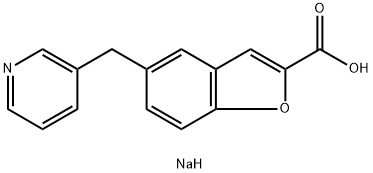 85666-17-7 結(jié)構(gòu)式
85666-17-7 結(jié)構(gòu)式
基本信息
中文別名
化合物 T11339化合物FUREGRELATE SODIUM
英文別名
U-63557Afuregralate sodium
SODIUM FUREGRELATE
FUREGRELATE SODIUM
FUREGRELATE SODIUM SALT
Furegrelate Sodium (U-63557A)
Sodium 5-(3-pyridinylmethyl)-2-benzofurancarboxylate
5-(3'-Pyridinylmethyl)benzofuran-2-carboxylate sodium
sodium:5-(pyridin-3-ylmethyl)-1-benzofuran-2-carboxylate
5-(3-Pyridylmethyl)benzofuran-2-carboxylic acid sodium salt
物理化學(xué)性質(zhì)
儲(chǔ)存條件−20°C
溶解度DMF: >25 mg/ml; DMSO: >21 mg/ml; Ethanol: >14.5 mg/ml; PBS pH 7.2: >16.5 mg/ml
形態(tài)白色至類白色結(jié)晶固體。
顏色White to light yellow
水溶解性Soluble in water at approximately 15mg/ml
U-63557A價(jià)格(試劑級(jí))
| 報(bào)價(jià)日期 | 產(chǎn)品編號(hào) | 產(chǎn)品名稱 | CAS號(hào) | 包裝 | 價(jià)格 |
| 2024/11/08 | HY-106080A | U-63557A Furegrelate sodium | 85666-17-7 | 5mg | 600元 |
| 2024/11/08 | HY-106080A | U-63557A Furegrelate sodium | 85666-17-7 | 10 mM * 1 mLin DMSO | 660元 |
| 2024/11/08 | HY-106080A | U-63557A Furegrelate sodium | 85666-17-7 | 10mg | 1000元 |
常見問題列表
生物活性
Furegrelate Sodium (U-63557A) 是一種有效的、口服的、選擇性的血栓素合酶 (thromboxane synthase) 抑制劑。Furegrelate Sodium 抑制人血小板微粒體血栓素 A2 (TxA2) 合酶,其 IC50 為 15 nM。Furegrelate Sodium 正在開發(fā)作為抗血小板藥物。體內(nèi)研究
Furegrelate Sodium (U-63557A) (1-5 mg/kg; Oral) prevents blockage of the coronary artery.
Furegrelate Sodium (0.1-5 mg/kg; i.v.) prevents the blockage of stenosed coronary arteries caused platelet aggregation.
Furegrelate Sodium blunts the development of hypoxia-induced pulmonary arterial hypertension (PAH) in an established neonatal piglet model primarily by preserving the structural integrity of the pulmonary vasculature.
Furegrelate also is orally available, has a long half-life of 4.2–5.8 hours in adult humans (compared to several other therapies for PAH including nitric oxide and prostacyclin analogs), and reportedly is highly specific for its target enzyme.
| Animal Model: | Mongrel dogs (19-30 kg) |
| Dosage: | 1-5 mg/kg |
| Administration: | Oral (via a gastric tube) |
| Result: | Prevented blockage of the coronary artery. |