72679-47-1
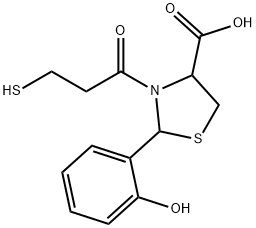 72679-47-1 結(jié)構(gòu)式
72679-47-1 結(jié)構(gòu)式
基本信息
Rentiapril (racemate)
2-(2'-Hydroxyphenyl)-3-(3-mercaptopropanoyl)-4-thiazolidine carboxylic acid
2-(2-hydroxyphenyl)-3-(3-sulfanylpropanoyl)-1,3-thiazolidine-4-carboxylic acid
4-Thiazolidinecarboxylic acid, 2-(2-hydroxyphenyl)-3-(3-mercapto-1-oxopropyl)-
物理化學(xué)性質(zhì)
常見(jiàn)問(wèn)題列表
Angiotensin converting enzyme (ACE)
A three-months toxicity study of an angiotensin converting enzyme (ACE) inhibitor, Rentiapril (CAS 80830-42-8), is performed in Sprague-Dawley rats by oral administration. The dose levels of 0, 30, 125, 500 and 1000 mg/kg are tested in both sexes, in which each experimental group comprised 10 rats. Another ACE inhibitor, captopril, is used as a reference compound. Rentiapril at the highest dose of 1000 mg/kg causes low food consumption and death of some animals with signs of bloody feces and anemia. In males and females receiving 500 and 1000 mg/kg, there are low body weight gain, increases in water intake, urine volume and serum BUN level, and decreases in levels of various erythrocytic parameters. Kidney weight is increased dose-dependently in both sexes. Histopathologically, renal changes in the 500 and 1000 mg/kg groups consist of proximal tubular degeneration, juxtaglomerular cell hyperplasia and interstitial cell infiltration. Similar, but mild, changes in proximal tubules are present in the female 125 mg/kg group. Dead animals from the highest dose groups further show gastrointestinal hemorrhagic erosion and/or ulcer, decrease bone marrow erythropoiesis and hepatocytic vacuolar degeneration. There is no pathological alteration in rats from other Rentiapril-treated groups, as well as in controls. These results indicate that the no-effect dose of Rentiapril in rats by three months oral administration is 30 mg/kg in female and 125 mg/kg in male.