62289-81-0
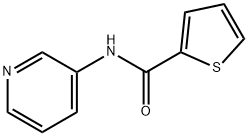 62289-81-0 結(jié)構(gòu)式
62289-81-0 結(jié)構(gòu)式
基本信息
N-pyridin-3-ylthiophene-2-carboxamide
2-Thiophenecarboxamide, N-3-pyridinyl-
物理化學(xué)性質(zhì)
常見問題列表
SW106065 (Compound 21, Cpd21) inhibits the human MPNST cell lines growth in a dose-dependent manner, and EC
50
concentrations of 439 nM and 753.6 nM for S462 and SNF96.2 cells, respectively. SW106065 remains nontoxic to normally dividing Schwann cells or mouse embryonic fibroblasts.
SW106065 (Cpd21; 0.25-5 μM; 24 hours; sMPNST cells) treatment shows a decreased percentage of cells in S phase, and a corresponding increased percentage in G1/G0 and G2/M.
SW106065 (Cpd21; 0.25-5 μM; 24 hours; sMPNST cells) treatment decreases the levels of cyclin A2, cyclin B1, cyclin D1, cyclin E, cdk4, and cdk6. And increases levels of cdkn1a and cdkn2a mRNA were observed in a dose-dependent manner.
SW106065 (Cpd21; 0.25-5 μM; 24 hours; sMPNST cells) treatment decreases the levels of Cyclin D1 protein.
SW106065 (Cpd21) treatment significant increase in the percentage of apoptotic cells.
Cell Cycle Analysis
| Cell Line: | sMPNST cells |
| Concentration: | 0.25 μM, 0.5 μM, 1 μM, 2.5 μM, and 5 μM |
| Incubation Time: | 24 hours |
| Result: | Showed a decreased percentage of cells in S phase, and a corresponding increased percentage in G1/G0 and G2/M. |
RT-PCR
| Cell Line: | sMPNST cells |
| Concentration: | 0.25 μM, 0.5 μM, 1 μM, 2.5 μM, and 5 μM |
| Incubation Time: | 24 hours |
| Result: | Decreased levels of cyclin A2, cyclin B1, cyclin D1, cyclin E, cdk4, and cdk6. Increased levels of cdkn1a and cdkn2a mRNA were observed in a dose-dependent manner. |
Western Blot Analysis
| Cell Line: | sMPNST cells |
| Concentration: | 0.25 μM, 0.5 μM, 1 μM, 2.5 μM, and 5 μM |
| Incubation Time: | 24 hours |
| Result: | Decreased levels of Cyclin D1 protein. |
SW106065 (Cpd21; 40 mg/kg; intraperitoneal injection; twice per day for 4 weeks) treatment can be delivered to mice in concentrations to sufficiently penetrate sMPNST tissue, and inhibit tumor development.
| Animal Model: | NCR-nu/nu female mice (6-7 week old) injected with MPNST cells |
| Dosage: | 40 mg/kg |
| Administration: | Intraperitoneal injection; twice per day for 4 weeks |
| Result: | Reduced MPNST burden in a mouse allograft model. |
