160472-97-9
中文名稱
N-[(1S,5R)-3,9-DIMETHYL-3,9-DIAZABICYCLO[3.3.1]NONAN-7-YL]-1H-INDAZOLE-3-CARBOXAMIDE;DIHYDROCHLORIDE
英文名稱
N-[(1S,5R)-7,9-dimethyl-7,9-diazabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide dihydrochloride
CAS
160472-97-9
分子式
C17H25Cl2N5O
分子量
349.86
MOL 文件
160472-97-9.mol
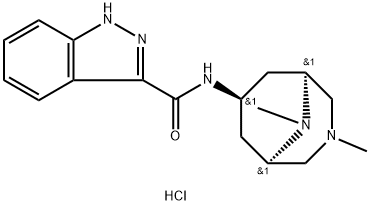 160472-97-9 結構式
160472-97-9 結構式
基本信息
中文別名
化合物 T27607 英文別名
N3389TN 3389T
N-3389T
Sinseron
Indisetron hydrochloride
Indisetron Dihydrochloride
N-[(1S,5R)-3,9-dimethyl-3,9-diazabicyclo[3.3.1]nonan-7-yl]-1H-indazole-3-carboxamide
N-[(1S,5R)-7,9-dimethyl-7,9-diazabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide dihydrochloride
N-[(1S,5R)-3,9-DIMETHYL-3,9-DIAZABICYCLO[3.3.1]NONAN-7-YL]-1H-INDAZOLE-3-CARBOXAMIDE
DIHYDROCHLORIDE
應用領域
用途一
Concomitant with the emesis provoked by radiation and cytotoxic drugs is an
increase in 5-hydroxytryptamine (5-HT) concentration in the brain stem and intestinal
mucosa, which in turn stimulates the 5-HT3 receptors on the adjacent vagal
afferent nerves. The depolarization of the vagal afferents is responsible for inducing the vomiting reflex. Indisetron, a 5-HT3/5-HT4 antagonist, has, therefore, been
launched in Japan as an anti-emetic. Since 5-HT4 is implicated in intestinal motility,
dual antagonism should improve the anti-emetic effect, although this remains to be
demonstrated. The diazabicycloamine portion of indisetron is prepared in four steps
starting with methylamine and bromoacetaldehyde dimethylacetal. Coupling to the
indazole core is accomplished via the acid chloride of 1H-indazole-3-carboxylic acid.
In vitro, indisetron displaced [3H]GR-65630 binding to the 5-HT3 receptor in rat brain
membranes in a concentration-dependent manner with a pKi value of 8.77, which is
comparable to the activities of other 5-HT3 antagonists such as granisetron and ondansetron.
In animal models (ferrets and dogs), indisetron reduced the number and
duration of cisplatin-induced emetic episodes when administered orally at 0.1–1mg/kg
prior to cisplatin treatment. Compared to granisetron and ondansetron, there were no
significant differences in the frequency of vomiting; however, the duration was significantly
reduced with indisetron. After the administration of anticancer agents, including
cisplatin, in phase II and phase III clinical trials, indisetron prevented vomiting
in 62%of patients (67 of 108) for 24 h. It faired even better in a phase III clinical study
where the anticancer agents did not include cisplatin; emesis was averted in 34 of 40
patients (85%). In a single oral dose study (4, 8, 16, and 32mg under fasting conditions
or a 16mg dose post-prandial) in healthy males, indisetron showed high oral
bioavailability (nearly 100%), and Cmax and AUC increased in a dose-dependent
manner. While four subjects experienced adverse effects, they were considered mild
and not clinically significant. In a phase I study comprised of 16mg of indisetron
administered b.i.d. over 7 consecutive days, the adverse reactions were also deemed
clinically insignificant although it was noted that two patients presented with constipation
and an increase in serum amylase. Steady state plasma levels were achieved
by day 3. Repeated administration did not change the pharmacokinetics
and accumulation of indisetron. Indisetron is metabolized by a host of CYP enzymes
(1A1, 2C9, 2D6, and 3A4) in the liver; however, it is unlikely to cause drug interactions
at clinical doses because the plasma concentrations are lower than those necessary for
CYP inhibition. Finally, as a 5-HT3 antagonist, indisetron should avoid the adverse
effects of the current dopamine-blocking anti-emetics, such as, sedation, ataxia, diarrhea,
and tasikinesia, making it a viable alternative to existing therapy.