140896-21-5
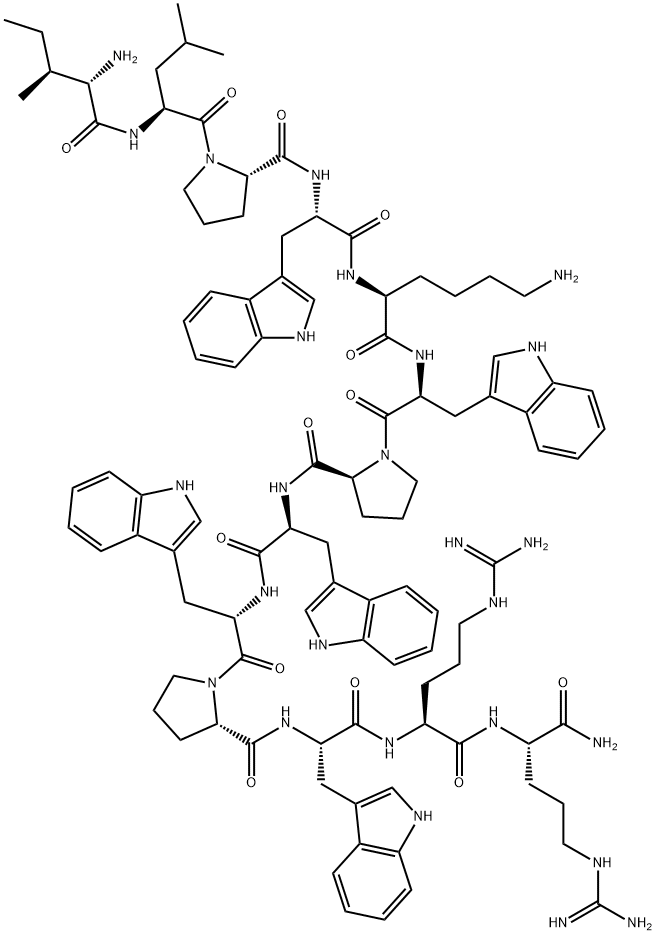 140896-21-5 結構式
140896-21-5 結構式
基本信息
INDOLICIDIN肽
Indolicidin TFA
ILPWKWPWWPWRR-NH2
IndolicidinIndolicid
Indolicidin, ≥97% (HPLC)
42: PN: US7381704 SEQID: 42 claimed sequence
ILE-LEU-PRO-TRP-LYS-TRP-PRO-TRP-TRP-PRO-TRP-ARG-ARG-NH2
H-ILE-LEU-PRO-TRP-LYS-TRP-PRO-TRP-TRP-PRO-TRP-ARG-ARG-NH2
L-ArgininaMide,L-isoleucyl-L-leucyl-L-prolyl-L-tryptophyl-L-lysyl-L-tryptophyl-L-prolyl-L-tryptophyl-L-tryptophyl-L-prolyl-L-tryptophyl-L-arginyl-
物理化學性質(zhì)
| 報價日期 | 產(chǎn)品編號 | 產(chǎn)品名稱 | CAS號 | 包裝 | 價格 |
| 2024/01/25 | HY-P0261 | ILE-LEU-PRO-TRP-LYS-TRP-PRO-TRP-TRP-PRO-TRP-ARG-ARG-NH2 Indolicidin | 140896-21-5 | 500μg | 1600元 |
| 2024/01/25 | HY-P0261 | ILE-LEU-PRO-TRP-LYS-TRP-PRO-TRP-TRP-PRO-TRP-ARG-ARG-NH2 Indolicidin | 140896-21-5 | 1mg | 2600元 |
| 2024/01/25 | HY-P0261 | ILE-LEU-PRO-TRP-LYS-TRP-PRO-TRP-TRP-PRO-TRP-ARG-ARG-NH2 Indolicidin | 140896-21-5 | 5mg | 7800元 |
常見問題列表
Indolicidin is comprised of 13 amino acids, 5 of which are tryptophan residues, and the carboxylterminal arginine is carboxamidated. Indolicidin has the highest tryptophan content of any known protein. The multiple tryptophan residues may play an important role in the function of this unique antibiotic peptide. Indolicidin is a tridecapeptide amide which possesses in vitro bactericidal activities comparable with the most active of the defensin or bactenecin peptides. Indolicidin binds purified surface lipopolysaccharide with high affinity and permeabilized the outer membrane of Escherichia coli to the small hydrophobic molecule 1-N-phenylnapthylamine (Mr 200), results consistent with indolicidin crossing the outer membrane via the self-promoted uptake pathway. The methyl esterification of indolicidin's carboxyl terminus increases its activity for Gram-negative and Gram-positive bacteria. In Gram-negative bacteria this is associated with an increased binding to lipopolysaccharide and increased permeabilization of the outer membrane. The cytoplasmic membrane is the site of action of indolicidin as assayed in Escherichia coli by the unmasking of cytoplasmic beta-galactosidase due to membrane permeabilization.