127308-98-9
中文名稱
Zamifenacinfumarate
英文名稱
Zamifenacinfumarate
CAS
127308-98-9
分子式
C27H29NO3.C4H4O4
分子量
531.602
MOL 文件
127308-98-9.mol
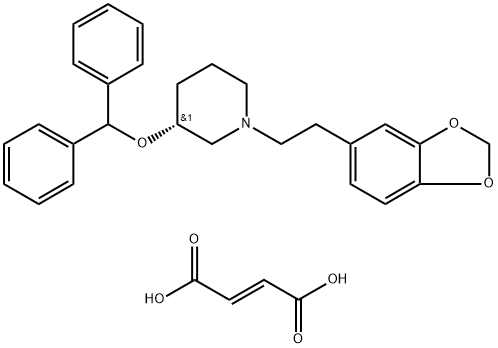 127308-98-9 結構式
127308-98-9 結構式
基本信息
中文別名
扎非那星富馬酸鹽 英文別名
UK-76654 fumarateZamifenacinfumarate
(3R)-1-[2-(1-,3-Benzodioxol-5-yl)ethyl]-3-(diphenylmethoxy)piperidinefumarate
物理化學性質
儲存條件Inert atmosphere,2-8°C
溶解度Soluble to 100 mM in DMSO and to 25 mM in ethanol
形態(tài)Powder
顏色White to off-white
Zamifenacinfumarate價格(試劑級)
| 報價日期 | 產品編號 | 產品名稱 | CAS號 | 包裝 | 價格 |
| 2024/11/08 | HY-107649 | Zamifenacinfumarate Zamifenacin fumarate | 127308-98-9 | 5mg | 1000元 |
| 2024/11/08 | HY-107649 | Zamifenacinfumarate Zamifenacin fumarate | 127308-98-9 | 10mM * 1mLin DMSO | 1170元 |
| 2024/11/08 | HY-107649 | Zamifenacinfumarate Zamifenacin fumarate | 127308-98-9 | 10mg | 1500元 |
常見問題列表
生物活性
Zamifenacin fumarate (UK-76654 fumarate) 是一種有效的腸道選擇性毒蕈堿 M3 受體拮抗劑。Zamifenacin 可顯著降低腸易激綜合癥的結腸蠕動。靶點
Muscarinic M3 receptor
體內研究
Zamifenacin exhibits moderate oral bioavailability (mouse 26%, rat 64%, dog 100%) and C
max
(mouse 92, rat 905, dog 416 ng/mL) following oral administration (mouse 13.2, rat 20 and, dog 5 mg/kg).
Zamifenacin exhibits terminal elimination half-lives (mouse 2.1, rat 6.0 and, dog 1.1 h) due to high plasma clearance (68, 35, and 39 mL/min/kg respectively combined with large volumes of distribution (12.5, 19.0, and 3.5 L/kg respectively) following intravenous administration (mouse 5.3, rat 5.0 and, dog 1.0 mg/kg).
| Animal Model: | Male CDl mice (mean weight 23 g) |
| Dosage: | 5.3 mg/kg for i.v.; 13.2 mg/kg for oral (Pharmacokinetic Analysis) |
| Administration: | Intravenous administration and oral administration |
| Result: | Oral bioavailability (26%), C max (92 ng/mL), T 1/2 (1.1 h). |
| Animal Model: | Male and female CD rats (mean weight 210 g) |
| Dosage: | 5.0 mg/kg for i.v.; 20 mg/kg for oral (Pharmacokinetic Analysis) |
| Administration: | Intravenous administration and oral administration |
| Result: | Oral bioavailability (64%), C max (905 ng/mL), T 1/2 (6.0 h). |
| Animal Model: | Male and two female beagle dogs (13-16 kg) |
| Dosage: | 1.0 mg/kg for i.v.; 5 mg/kg for oral (Pharmacokinetic Analysis) |
| Administration: | Intravenous administration and oral administration |
| Result: | Oral bioavailability (100%), C max (416 ng/mL), T 1/2 (1.1 h). |
