| Identification | Back Directory | [Name]
Flupyradifurone | [CAS]
951659-40-8 | [Synonyms]
Flupyradifurone
Flupyradifurone Solution
4-[(6-Chloro-3-pyridylmethyl)(2,2-difluoroethyl)amino]furan-2(5H)-one
4-(((6-Chloropyridin-3-yl)methyl)(2,2-difluoroethyl)amino)furan-2(5H)-one
2(5H)-Furanone, 4-[[(6-chloro-3-pyridinyl)methyl](2,2-difluoroethyl)amino]-
4-{[(6-chloropyridin-3-yl)methyl](2,2-difluoroethyl)
amino}-2,5-dihydrofuran-2-one | [Molecular Formula]
C12H11ClF2N2O2 | [MDL Number]
MFCD22373627 | [MOL File]
951659-40-8.mol | [Molecular Weight]
288.68 |
| Hazard Information | Back Directory | [Description]
Flupyradifurone(FPF) is a novel butenolide insecticide developed for foliar, soil and seed treatment applications (Nauen et al., 2015). FPF was commercially introduced to the market in 2014 as an integrated pest management (IPM)-friendly tool (Bordini et al., 2021), and is registered for use in a wide variety of fruit and vegetable crops and defined broad acre crops. It targets some of the world′s most destructive sucking pests including aphids, psyllids, scales, leafhoppers, mealy bugs, and is particularly important for the control of whiteflies such as Bemisia tabaci, a vector of serious phytopathogenic viruses such as tomato yellow leaf curl virus and cucurbit yellow stunting disorder virus (Castle et al., 2017; Roditakis et al., 2017). | [Uses]
Flupyradifurone, can be used as an insecticide. | [Definition]
ChEBI: Flupyradifurone is a tertiary amino compound that is ammonia in which the nitrogens have been replaced by (6-chloropyridin-3-yl)methyl, 2,2-difluoroethyl, and 5-oxo-2,5-dihydrofuran-3-yl groups, respectively. A nicotinic acetylcholine receptor (AChR) agonist, it is used as an insecticide to control sucking pests in a variety of crops. It has a role as a nicotinic acetylcholine receptor agonist and an insecticide. It is a butenolide, a monochloropyridine, an organofluorine insecticide, an enamine and a tertiary amino compound. | [Preparation]
Two different pathways for the synthesis of flupyradifurone
Starting from tetronic acid, one approach consists in two consecutive reactions. Where the tetronic acid reacts firstly with a difluoroethane-1-amine and secondly with 2-Chloro-5- (chloromethyl)pyridine.
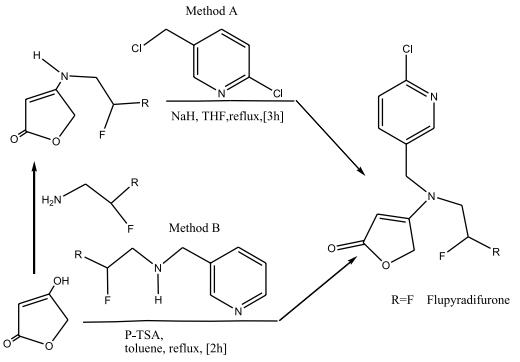
And the other approach, where the tetronic acid reacts with difluoroethane-1-amine derivative in the presence of 4-toluenesulfonic acid in a “one pot” approach to yield flupyradifurone. | [storage]
4°C, protect from light |
|
|