| Identification | Back Directory | [Name]
BenzylVinylEther | [CAS]
935-04-6 | [Synonyms]
BenzylVinylEther
Vinyl benzyl ether
1-(Benzyloxy)ethene
Benzyl ethenyl ether
Vinyloxymethyl-benzene
[(ethenyloxy)methyl]benzene
Benzene,[(ethenyloxy)methyl]- | [Molecular Formula]
C9H10O | [MDL Number]
MFCD12547209 | [MOL File]
935-04-6.mol | [Molecular Weight]
134.18 |
| Chemical Properties | Back Directory | [Boiling point ]
183-184 °C | [density ]
0.9710 g/cm3 | [Fp ]
68 °C | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
liquid | [color ]
Colourless | [Specific Gravity]
0.97 | [InChI]
InChI=1S/C9H10O/c1-2-10-8-9-6-4-3-5-7-9/h2-7H,1,8H2 | [InChIKey]
AZDCYKCDXXPQIK-UHFFFAOYSA-N | [SMILES]
C1(COC=C)=CC=CC=C1 |
| Hazard Information | Back Directory | [Uses]
Benzyl vinyl ether is an organic raw material intermediate compound used in the synthesis of 2-(Benzyloxy)acetaldehyde and 3-(Benzyloxy)cyclobutanone. | [Application]
A crystalline poly(benzyl vinyl ether) (PBVE) was obtained by the polymerization of benzyl vinyl ether (BVE).
The benzyl vinyl ether polymerization has been investigated in toluene by Et3Al-TiCJ4 catalyst. When the catalyst preparation and the monomer addition were carried out at 0°C, and the reaction mixture was brought to 60°C, an insoluble polymeric product was obtained with poly(benzyl vinyl ether) (PBVE). This was formed by the reaction of PBVE and TiC14 in the catalyst system and the subsequent hydrolysis of the reaction product.
The living cationic polymerization of benzyl vinyl ether was achieved by the CH3CH(OiBu)OCOCHil)/EtAICl2 initiating system in the presence of added bases. Polymerizations in the presence of ethyl acetate at below 0°C in a non-polar solvent (toluene or CCl4 ) gave living polymers with a narrow molecular weight distribution (MWD) (Mw/M. - 1.1)[1-2].
| [Synthesis]
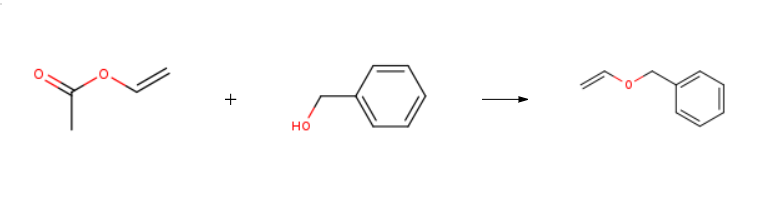
Benzyl vinyl ether is prepared by the reaction of vinyl acetate and benzyl alcohol. The steps are as follows:
Benzyl alcohol (5.18 mL, 50.0 mmol) and vinyl acetate (9.22 mL, 100 mmol, 2.0 equiv) are added to a suspension of [Ir(cod)Cl]2 (336 mg, 0.500 mmol, 1 mol %) and Na2CO3 (3.18 g, 30.0 mmol, 0.6 equiv) in toluene (50 mL).
After stirring for 2 hours at 100° C., the mixture obtained is cooled down to ambient temperature and filtered on Celite.
The filtrate is concentrated under reduced pressure and the residue purified by flash chromatography on silica gel (petroleum ether/EtOAc: 98/2) in order to produce a yellow oil (5.15 g, 77%).
1H-NMR (CDCl3, 400 MHz) δ 7.39-7.29 (m, 5H), 6.57 (dd, J=14.3 Hz and J=6.8 Hz, 1H), 4.76 (s, 2H), 4.30 (dd, J=14.3 Hz and J=2.0 Hz, 1H), 4.08 (dd, J=6.8 Hz and J=2.0 Hz, 1H).
 | [References]
[1] Sadahito Aoshima, Eiichi Kobayashi, Shigeyuki Iwasa. “Living Cationic Polymerization of Benzyl Vinyl Ether and Its Block Copolymers with Narrow Molecular Weight Distribution.” Polymer Journal 26 8 (1994): 912–919.
[2] Heimei Yuki. “Polymerization of Benzyl Vinyl Ether with a Ziegler Catalyst and Reaction of Poly(benzyl vinyl ether) with TiCl4.” Polymer Journal 1 3 (1970): 271–277.
|
|
|