| Identification | Back Directory | [Name]
LHRH | [CAS]
9034-40-6 | [Synonyms]
LRF
lrh
gnrh
LHRH
gn-rh
lh-rf
ay24034
rHuLHRH
luliberin
LHRH, HUMAN
LH-RH ACETATE
luteostimulin
gonadoliberin
LHRH (SEA BREAM)
PYR-HWSYGLRPG-NH2
LHRH (LAMPREY III)
lh-releasingfactor
lh-releasinghormone
GNRH (PORCINE, RAT)
GONADORELINE, HUMAN
GONADOLIBERIN ACETATE
Luliberin,LHRH, human
lh-fshreleasinghormone
LH-RH (HUMAN) PORCINE, RAT
gonadotropin-releasingfactor
lh-fshreleasinghormone)a(10)-
GONADOTROPIN-RELEASING HORMONE
LUTEINISING HORMONE RELEASING HORMONE
LEUTINIZING HORMONE RELEASING HORMONE
LUTEINIZING HORMONE RELEASING HORMONE
GONADOTROPIN RELEASING HORMONE ACETATE
GONADOTROPIN-RELEASING HORMONE (SEA BREAM)
LUTENIZING HORMONE RELEASING HORMONE HUMAN
LUTEINIZING HORMONE RELEASING HORMONE HUMAN
LH-RH LUTEINIZING HORMONE-RELEASING HORMONE
PYR-HIS-TRP-SER-HIS-ASP-TRP-LYS-PRO-GLY-NH2
GLP-HIS-TRP-SER-TYR-GLY-LEU-ARG-PRO-GLY-NH2
PYR-HIS-TRP-SER-TYR-GLY-LEU-ARG-PRO-GLY-NH2
GLP-HIS-TRP-SER-TYR-GLY-LEU-SER-PRO-GLY-NH2
PGLU-HIS-TRP-SER-TYR-GLY-LEU-ARG-PRO-GLY-NH2
GONADOTROPIN-RELEASING HORMONE (LAMPREY III)
LUTEINIZING HORMONE-RELEASING HORMONEACETATE
GONADOTROPIN-RELEASING HORMONE (PORCINE, RAT)
PYROGLU-HIS-TRP-SER-TYR-GLY-LEU-ARG-PRO-GLY-NH2
LUTEINIZING HORMONE-RELEASING FACTOR (SEA BREAM)
LUTEINIZING HORMONE-RELEASING HORMONE (SEA BREAM)
LUTEINIZING HORMONE-RELEASING FACTOR (LAMPREY III)
Luteinizing hormone releasing hormone acetate salt
LUTEINIZING HORMONE-RELEASING HORMONE (LAMPREY III)
LUTEINIZING HORMONE-RELEASING HORMONE (LH-RH), HUMAN
PGLU-HIS-TRP-SER-TYR-GLY-LEU-ARG-PRO-GLY-NH2 ACETATE
LUTEINIZING HORMONE RELEASING HORMONE (HUMAN) PORCINE, RAT
LUTEINIZING HORMONE-RELEASING HORMONE (L HRH) SYNTHETIC >9
Recombinant Human Leutenizing hormone Releasing Hormone(Gonadorelin) | [EINECS(EC#)]
232-895-0 | [Molecular Formula]
C55H75N17O13 | [MDL Number]
MFCD00167538 | [MOL File]
9034-40-6.mol | [Molecular Weight]
1182.29 |
| Questions And Answer | Back Directory | [Discovery]
In 1971, GnRH was first isolated from the porcine
and ovine by Schally1 and Guillemin, respectively.
In nonmammalian vertebrates, GnRH was isolated
from the chicken in 1982 and the salmon in 1983. To
date, 15 different isoforms of GnRHs have been identified in vertebrates. In nonvertebrate chordates, multiple GnRH-like peptides have been identified in
tunicates, first from Chelyosoma productum in 1996. | [Structure]
Vertebrate GnRH isoforms consist of 10 aa residues
with a pyroglutamic acid at the N-terminus and an amidated Gly at the C-terminus . Based on the primary structure, phylogenetic analysis, and synteny,
multiple GnRH forms are classified into three paralogous
groups: GnRH1, GnRH2, and GnRH3.3, 4 The peptides
are bent with a β-turn around Gly6
, and the N-terminal
and C-terminal amino acid residues are important for
binding to the GnRH receptor (GnRH-R).5
Primary structure
The primary structure of GnRH is highly conserved in
the N-terminus (positions 1–4) and in the C-terminus
(positions 9–10). Position 8 is the most variable.
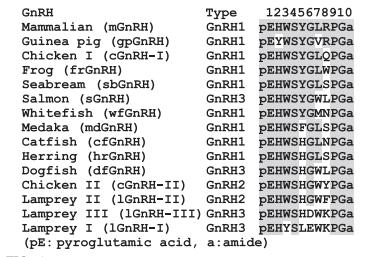 | [Properties]
Mr 1050–1250. GnRH is inactivated in 0.9M HCl at
100°C for 60min, and is also inactivated by endopeptidases such as chymotrypsin and papain. mGnRH
and cGnRH-I are soluble in water; cGnRH-II and
sGnRH are insoluble in water but soluble in 10mM
acetic acid. | [Receptors]
GnRH-R is a membrane-bound GPCR belonging to the
Class A (rhodopsin-like) subfamily. Two major types of
GnRH-Rs—type I and type II—have been identified in
vertebrates.The type I GnRH-Rs, present
in mammals, coelacanth, and some cartilaginous fish,
lack a C-terminal intracellular domain that is responsible
for rapid desensitization, and show a strong preference
for GnRH1. The type II GnRH-Rs, present in both mam�malian and nonmammalian species, show a preference
for GnRH2. The type II GnRH-Rs are further divided into
two subtypes, IIa and IIb. In some mammals, including
humans, the type II GnRH-R is a nonfunctional receptor
encoded by a pseudogene. The human type I GnRH-R
consists of 328 aa residues. | [Clinical implications]
Idiopathic hypogonadotropic hypogonadism (IHH) is
a family of genetic disorders that is associated with
defects in the production and/or action of hypothalamic
peptide, which controls GnRH. As mentioned above,
IHH with anosmia is referred to as KS. In addition, the
most common cause of delayed puberty is a functional
defect in the production of GnRH from the
hypothalamus. | [Synthesis and release]
The regulation of GnRH secretion by internal and environmental factors such as growth, energy condition,
light, photoperiod, temperature, and social status is critical for reproductive success. Regulatory mechanisms of
GnRH1 neurons involve many stimulatory and inhibitory factors including gonadal steroids, neuropeptides,
and neurotransmitters (GABA, glutamate, norepinephrine). Kisspeptin stimulates GnRH1 secretion and has
key roles in the transmission of the negative and positive
feedback effects of gonadal steroids, the metabolic regulation, and the photoperiodic control of reproduction. Gonadotrophin-inhibitory hormone (GnIH) may inhibit
gonadotropin secretion by decreasing the activity of
GnRH1 neurons. |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
60 | [Safety Statements ]
53-45 | [HS Code ]
3504009000 | [Safety Profile]
An experimental
teratogen. Human reproductive effects in
women by subcutaneous route: menstrual
cycle changes and other unspecified effects.
Experimental reproductive effects. Used in
the treatment of oligospermia and male
inferthty. See also LUTEINIZING
HORMONE and other luteinizing
hormone-releasing hormone entries. | [Toxicity]
LD50 oral in rat: > 3gm/kg |
| Hazard Information | Back Directory | [Description]
Gonadotropin-releasing hormone is a decapeptide that is produced in neurosecretory
cells within the hypothalamus. GnRH stimulates the synthesis and release of luteinizing hormone (LH) and folliclestimulating hormone (FSH) from the anterior pituitary
and also controls reproductive behavior, thus serving as a
central regulator of reproduction in vertebrates. It is used
not only as a fertility drug but also as an antifertility drug. | [Definition]
ChEBI: Gonadorelin is a ten-membered synthetic oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, glycyl, leucyl, arginyl, prolyl and glycinamide residues joined in sequence. It has a role as a gonadotropin releasing hormone agonist. It is an oligopeptide and a peptide hormone. | [Indications]
GnRH (gonadorelin, luteinizing hormone–releasing
hormone) is a decapeptide that stimulates production
of LH and FSH. It is released in bursts from the hypothalamus
at regular intervals, about every 2 hours, although
in women the interval may lengthen in the luteal
end of the menstrual cycle.The pituitary gland responds
to these regular pulses by producing LH and FSH. The
pattern of LH and FSH in cycling women, including the
large burst of LH release before ovulation, can be stimulated
by regular administration of GnRH pulses. The
large burst of LH from the pituitary gland appears to be
induced by feedback through estradiol and other products
of the gonads that change the response of the pituitary
gland to the GnRH pulses rather than by large
changes in the amounts of GnRH secreted. The stimulatory
response to GnRH depends on pulsatile administration
and the timing of the pulses. Continual administration
of GnRH does not have the same effects as
pulsatile administration; although production of LH
and FSH is stimulated initially, it is suppressed within a
few days. Part of this desensitization to GnRH is caused
by a decrease in the number of pituitary receptors for
GnRH; additional postreceptor mechanisms are also
important in this complete suppression. | [Indications]
Oxytocin (Pitocin, Syntocinon) is a cyclic 8–amino acid
peptide that is synthesized in the paraventricular nucleus
of the hypothalamus and transported within hypothalamic
neurons (in association with neurophysin)
to the posterior pituitary for storage. Its mechanism of
action involves the direct stimulation of oxytocin receptors
found on the myometrial cells. Oxytocin circulates
unbound in the plasma, where it has a half-life of approximately
15 minutes. It is primarily inactivated in the
kidneys and liver.
Oxytocin (Pitocin, Syntocinon) causes milk release (letdown)
by stimulating contraction of the myoepithelial
cells of the milk ducts in lactating mammary glands; this
forces milk from the alveoli of the breast. Oxytocin release
is stimulated by suckling and by auditory and visual
stimuli, such as a baby’s cry.Oxytocin is available as
a nasal spray, which is used as an aid to lactation when
milk ejection is impaired. | [Biological Functions]
Gonadotropin-releasing hormone (GnRH) is a decapeptide that causes the release of
the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), from the
anterior pituitary gland, but not in equal amounts (FSH release is partially inhibited by the
gonadal protein inhibin). Therefore, GnRH is intimately involved in the control of both male and
female reproduction. Medicinal chemists have capitalized on the relatively simple decapeptide
structure of GnRH by preparing many analogues as potential fertility and antifertility agents,
several of which are commercially available, especially those that are referred to as
superagonists. It is known that GnRH can be degraded by enzymatic cleavage between Tyr5-
Gly6 and Pro9-Gly10. Structure–activity relationship studies of GnRH analogues have shown
that when Gly6 is replaced with certain D-amino acids, as well as with changes in the peptide C terminus, they generally are less susceptible to proteolytic enzymes, resulting in a longer lasting action. For that reason, they are referred to as superagonists. Furthermore, when these
D-amino acids at position 6 are hydrophobic, the half-life is enhanced. | [Mechanism of action]
In physiological doses, GnRH agonists are able to induce ovulation and spermatogenesis by increasing LH and FSH levels and the resulting sex steroid levels, as does the normal hormone. In larger pharmacological (therapeutic) doses, however, GnRH agonists, especially the superagonists, block implantation of the fertilized egg, cause luteolysis of the corpus luteum, and can act as postcoital contraceptive agents (although not approved for this latter use). This paradoxical antifertility effect seen with the superagonists has been attributed to the fact that GnRH must be administered in a low-dose, pulsatile manner for it to be therapeutically effective as a fertility agent. Natural GnRH release from the hypothalamus occurs in a pulsatile manner. When GnRH or, especially, a superagonist is administered in pharmacological doses each day, LH and FSH levels will initially rise but then begin to fall after a few days because of target tissue desensitization/downregulation of pituitary GnRH receptors. The continued use of these agents in a nonpulsatile manner will result in a drastic drop of the gonadal steroid levels to near castrate levels in both males and females, thereby giving rise to their use in such conditions as precocious puberty, endometriosis, and advanced metastatic breast and prostate carcinoma.Typically, however, the GnRH superagonists take approximately 2 weeks to finally desensitize
the GnRH receptors, and during this time, there is a transient rise in LH and FSH levels, which
often results in an initial “flare-up” of the original symptoms. | [Clinical Use]
Because mGnRH has a short half-life of several
minutes, a number of GnRH analogs with high potency
and long half-life have been synthesized. The effects of
GnRH and its analogs are dependent on the dose and
method of administration. Low doses of GnRH delivered
in a pulsatile fashion restore fertility in hypogonadal
patients. High doses of GnRH or continuous administration first results in an increase in LH and FSH secretion,
followed by a decrease in LH and FSH levels due to
desensitization, then by a decline in gonadal steroid
levels. The administration of antagonists interrupts
GnRH-dependent LH and FSH secretion through competition with endogenous GnRH, but the doses required are
much higher that the desensitizing agonists. mGnRH is
available as gonadorelin for veterinary use. Various
GnRH agonists are used in the treatment of hormoneresponsive cancers such as prostate and breast cancers,
estrogen-dependent conditions such as endometriosis,
and precocious puberty. They are also widely used in
ART (assisted reproductive technology including IVFET, in vitro fertilization, and embryo transfer) to block
the endogenous LH surge in the controlled ovarian
stimulation | [Clinical Use]
Oxytocin is generally considered to be the drug of
choice for inducing labor at term. In combination with
amniotomy, oxytocin is highly successful in inducing
and augmenting labor. When given oxytocin, approximately
80% of patients with documented labor disorders
progress into labor and deliver vaginally. It has also
been used following incomplete abortion after 20 weeks
of gestation (although use of prostaglandins may be
preferred in this instance), and it may be used after fullterm
delivery to prevent or control uterine hemorrhage.
Oxytocin in high doses is used to induce abortion. An
oxytocin challenge test (an assessment of the fetal heart
rate in response to oxytocin-induced contractions) can
be performed in certain high-risk (e.g., those with hypertension,
diabetes, preeclampsia) obstetrical patients
as a measure of fetal well-being. | [Side effects]
Inappropriate use of oxytocin can lead to uterine
rupture, anaphylactoid and other allergic reactions, and
possibly maternal death. Prolonged stimulation of uterine
contractions can result in the following fetal adverse
reactions: persistent uteroplacental insufficiency, sinus
bradycardia, premature ventricular contractions, other
arrhythmias, and fetal death. Prolonged use of oxytocin
can lead to water intoxication secondary to the antidiuretic
hormone–like effects of oxytocin. Maternal and
fetal cardiovascular parameters should be monitored
during oxytocin administration. |
|
|