| Identification | Back Directory | [Name]
Poly(vinyl alcohol) | [CAS]
9002-89-5 | [Synonyms]
PVA
BP17
BP05
Alvyl
Covol
GH 20
GL 02
GL 03
GLO 5
GM 14
Kurat
Lemol
NH 18
NM 11
NM 14
Poval
PVS 4
Vinol
Mowiol
EP 160
FH 1500
Alkotex
Elvanol
M 13/20
Vinalak
Vinarol
PVA 008
PVA-103
PVA-224
PVA-203
PVA-210
PVA-105
PVA-124
PVA-117
vinylon
PVA-RAFT
Vinarole
Polyviol
Enbra OV
Gelvatol
Gohsenol
Covol 971
Poval 117
Poval 120
Poval 203
Poval 205
Poval 217
Poval 420
Polyvinol
Polydesis
VPB 105-2
Rhodoviol
Sloviol R
Vinol 125
Vinol 205
Vinol 351
Vinol 523
PVA Fiber
PVAFIBRES
PVACP1000
Elvanol HV
Vinarol DT
Vinarol ST
PVAL 45/02
PVAL 55/12
Vinacol MH
Lemol 5-88
Lemol 5-98
Poval C 17
Poval 217S
Poval 205S
Poval 1700
Aracet APV
Kuralon VP
Kurare 217
Mowiol®
Lemol 12-88
Lemol 16-98
Lemol 24-98
Lemol 30-98
Lemol 51-98
Lamephil OJ
Gohsenol GH
Lemol 60-98
Lemol 75-98
Lemol GF-60
Mowiol?3-96
Mowiol?4-98
n=1750+/-50
Mowiol20-98
GOHSENOLGLO3
GOHSENOLGLO5
Mowiol? 3-98
Mowiol?40-88
Mowiol?28-99
Sumitex H 10
Mowiol 26-88
Elvanol 5105
Elvanol T 25
Elvanol 90-50
Gelvatol 1-30
Gelvatol 1-60
Gelvatol 1-90
Galvatol 1-60
Gelvatol 2060
Gelvatol 2090
Gelvatol 3-91
Elvanol 50-42
Elvanol 52-22
Elvanol 70-05
Elvanol 71-30
Cipoviol W 72
Alcotex 17F-H
Alcotex 88/05
Alcotex 88/10
Alcotex 99/10
Gosenol KH-17
Polysizer 173
Mowiol? 56-98
KARTONOLAW-12
Mowiol(R) 4-98
Mowiol(R) 8-88
Mowiol N 30-88
Mowiol N 50/88
Mowiol N 50-98
Mowiol N 70-98
Vinavilol 2-98
Gohsenol NL 05
Gohsenol NM 14
Kurare PVA 205
Gohsenol GH 17
Gohsenol GH 20
Gohsenol GH 23
Gohsenol GL 03
Gohsenol GL 05
Gohsenol GL 08
Gohsenol GM 14
Gohsenol GM 94
Gohsenol KH 17
Gohsenol MG 14
Gohsenol N 300
Gohsenol NH 05
Gohsenol NH 17
Gohsenol NH 18
Gohsenol NH 20
Gohsenol NH 26
Elvanol 73125G
Elvanol 522-22
Elvanol 52-22G
Elvanol 51-05G
Gohsenol AH 22
Gelvatol 20-30
Gohsenol nk 114
Gohsenol GM 14L
Gohsenol NM 114
Gtohsenol GL 05
Rhodoviol 4/125
PVA (PVOH) FILM
Polyvinyl glycol
Rhodoviol 4-125P
Rhodoviol 16/200
Polyviol W 28/20
Kurare Poval 120
Kurare Poval 1700
Polyviol W 40/140
Polyviol M 13/140
Polyviol MO 5/140
Polyviol W 25/140
Rhodoviol R 16/20
Vinylon Film 2000
Vinylon Film 3000
Warcopolymer A 20
Mowiol[R] PVA-103
Mowiol[R] PVA-105
Mowiol[R] PVA-117
Mowiol[R] PVA-124
Mowiol[R] PVA-203
Mowiol[R] PVA-210
Mowiol[R] PVA-224
Poly(vinyl alcohol)
POLYVINYLIC ALCOHOL
POLYVINYLALCOHOL,USP
Pva(PolyvinylAlcohol)
POLYVINYLALCOHOLFIBRE
Polyvinyl alcohol 124
Poly(vinyl alcohol)224
EZNA KIT BACTERIAL DNA
POLYVINYLALCOHOLFIBRES
POLYVINYL ALCOHOL 3-96
Polyvinyl Alcohol Film
POLYVINYL ALCOHOL 2000
Vinylon Film VF-A 2500
polyvinyl alcohol 3-98
POLYVINYL ALCOHOL (PVA)
Vinyl alcohol, polymers
Poly(1-hydroxyethylene)
Polyvinyl Alcohol Fiber
PVA (polyvinil alcohol)
Polyvinyl alcohol 17-99
Poly(vinyl alcohol) 105
Poly(vinyl alcohol) 205
Polyvinyl alcohol AH-26
Polyvinyl alcohol 26-88
Poly(vinyl alcohol) 1795
Poly(vinyl alcohol) 1797
Poly(vinyl Alcohol)
POLYVINYL ALCOHOL USP/NF
POLYVINYL ALCOHOL 27'000
Polyvinyl alcohol 1750±50
Mowiol(R) 4-88 Mw ~31,000
Mowiol(R) 4-98 Mw ~27,000
Mowiol(R) 6-98 Mw ~47,000
Mowiol(R) 8-88 Mw ~67,000
Polyvinyl alcohol PVA-124
Mowiol(R) 10-98 Mw ~61,000
Polyvinyl Alcohol, Reagent
Polyvinyl Alcohol (100 mg)
Mowiol(R) 18-88 Mw ~130,000
Mowiol(R) 20-98 Mw ~125,000
Mowiol(R) 28-99 Mw ~145,000
Mowiol(R) 56-98 Mw ~195,000
Vinyl alcohol - polymerised
Polyvinyl Alcohol, 88-89%
Polyvinyl Alcohol, 99-100%
POLYVINYL ALCOHOL 99% POWDER
Polyvinyl Alcohol, hydrolized
Polyvinylalkohol, Homopolymer
Denki Kagaku Kogyo Denka Poval
POLYVINYL ALCOHOL 4-88, 31'000
Poly(vinyl Alcohol) n=1750+/-50
Mowiol 8-88,Poly(vinyl alcohol)
Mowiol 40-88,Poly(vinyl alcohol)
POLYVINYL ALCOHOL STANDARD 7'200
POLYVINYL ALCOHOL STANDARD 82'000
POLYVINYL ALCOHOL STANDARD 35'000
POLYVINYL ALCOHOL STANDARD 55'000
Polyvinyl alcohol (Release agent)
polyvinyl alcohol standard 200000
poly(vinyl alcohol) macromolecule
POLYVINYL ALCOHOL STANDARD 122'400
POLYVINYL ALCOHOL STANDARD 137'100
POLYVINYL ALCOHOL STANDARD 170'500
POLYVINYL ALCOHOL 98-99% HYDROLYZED
POLYVINYL ALCOHOL M.W. APP. 115,000
Polyvinyl alcohol, fully hydrolized
Parteck? SRP 80 (Polyvinyl alcohol)
Poly(vinyl alcohol), 75% Hydrolyzed
Polyvinyl alcohol, fully hydrolyzed
POLY(VINYL ALCOHOL), 87-90% HYDROLYZ
Poly(vinyl alcohol) 1788 low-viscosity
POLYVINYL ALCOHOL 145000 FOR SYNTHESIS
POLY(VINYL ALCOHOL), 87-90% HYDROLYZED
Poly(vinyl alcohol), 99-100% Hydrolyzed
Polyvinyl Alcohol, Partially Hydrolyzed
POLY(VINYL ALCOHOL), 99+% HYDROLYZED, V&
POLYVINYL ALCOHOL PROTECTIVE COLLOID FOR
Poly Vinyl Alcohol (various viscosities)
Mowiol(R) 40-88 average Mw ~205,000 g/Mol
Poly(vinyl alcohol) (Fully hydrolyzed-very
POLYVINYLALCOHOL,99-100%,HYDROLYZED,REAGENT
PVA
Vinyl alcohol - polymerised
Vinyl alcohol
POLY(VINYL ALCOHOL) (FULLY HYDROLYZED-LOW M.WT.)
Polyvinyl alcohol hydrolyzed, low molecular weight
Polyvinyl Alcohol, Hydrolyzed, Average M.W. 88,000
Poly(vinyl alcohol) Mw 9,000-10,000, 80% hydrolyzed
Polyvinyl alcohol, Low molecular weight, Hydrolyzed
Polyvinyl alcohol, High molecular weight, Hydrolyzed
Polyvinylalcohol,86-89%hydrolyzed,lowmolecularweight
Polyvinylalcohol,98-99%hydrolyzed,lowmolecularweight
POLYVINYL ALCOHOL AV. MOL. WT.*70,000-10 0,000 (LALL
Poly(vinyl alcohol) (Enzyme Grade)(Fully hydrolyzed)
POLYVINYL ALCOHOL, PROTECTIVE COLLOID FO R TITRATIONS
Polyvinylalcohol,87-89%hydrolyzed,highmolecularweight
Poly(vinyl alcohol) Mw 89,000-98,000, 99+% hydrolyzed
Poly(vinyl alcohol),75% hydrolyzed, average M.W. 2000
Polyvinyl Alcohol, 99-100 Percent, Hydrolyzed, Reagent
Poly(vinyl alcohol) Mw 85,000-124,000, 99+% hydrolyzed
Poly(vinyl alcohol), 75% hydrolyzed, approx. M.W. 2000
Polyvinyl alcohol, Medium molecular weight, Hydrolyzed
Poly(vinyl alcohol), 88% hydrolyzed, average M.W. 22000
Poly(vinyl alcohol), 88% hydrolyzed, average M.W. 88000
Poly(vinyl alcohol), 95% hydrolyzed, average M.W. 95000
Poly(vinyl alcohol), 98% hydrolyzed, average M.W. 16000
Poly(vinyl alcohol) Mw 13,000-23,000, 87-89% hydrolyzed
Poly(vinyl alcohol) Mw 146,000-186,000, 99+% hydrolyzed
Poly(vinyl alcohol) Mw 31,000-50,000, 98-99% hydrolyzed
Poly(vinyl alcohol) average Mw 130,000, 99+% hydrolyzed
Poly(vinyl alcohol), 99% hydrolyzed, average M.W. 86,000
Poly(vinyl acetate), cyanomethyl diphenylcarbamodithioate
Polyvinyl alcohol, low molecular weight, 98-99% hydrolyzed
Polyvinyl alcohol, high molecular weight, 98-99% hydrolyzed
Polyvinyl alcohol, high molecular weight, 87-89% hydrolyzed
Poly(vinyl alcohol), 88% hydrolyzed, average M.W. 88000 1KG
Poly(vinyl alcohol), 99-100% hydrolyzed, approx. M.W. 86000
Polyvinyl alcohol, 98-99% hydrolyzed, high molecular weight
Poly(vinyl alcohol), 88% hydrolyzed, average M.W. 88000 25GR | [EINECS(EC#)]
209-183-3 | [Molecular Formula]
C2H3 * | [MDL Number]
MFCD00677796 | [MOL File]
9002-89-5.mol | [Molecular Weight]
27.0452 |
| Questions And Answer | Back Directory | [Air & Water Reactions]
Water soluble.
| [Fire Hazard]
This chemical is combustible. The dusts of this chemical are a slight explosion hazard when exposed to flame. (NTP, 1992)
| [Health Hazard]
SYMPTOMS: Inhalation of the dust of this chemical may cause irritation of the nose and throat and cause coughing and chest discomfort if heated above 390° F. The dusts may also irritate the eyes. Implantation of this chemical into the breast has been associated with fibrosis.
ACUTE/CHRONIC HAZARDS: This compound may be harmful by ingestion and inhalation. It may cause irritation. When heated to decomposition it emits acrid smoke, irritating fumes and toxic fumes of carbon monoxide and carbon dioxide. (NTP, 1992)
| [Reactivity Profile]
Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73. 1967; J, Org. Chem. 28:1893. 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous- carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
| [Chemical properties]
Polyvinyl alcohol(9002-89-5) is a hydrolysis product of polyvinyl acetate, rather than by the polymerization of monomers; the molecular backbone contains 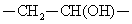 . The specific gravity of this product 1.25 to 1.35 and the melting point is 212 ~ 267 ℃. It is soluble in hot water and hot dimethyl sulfoxide. Animal experiments show that polyvinyl alcohol, without stimulation, causes no significant toxicity upon subcutaneous, intramuscular, intravenous injection. Polyvinyl alcohol resin products appear as white solid with the appearance of sub-floc, granular and powder; it is non-toxic, tasteless, non-polluting and is soluble in water of 80--90 ℃. Its aqueous solution has good adhesiveness and film-forming property; it can resist most organic solvents such as oils, lubricants and hydrocarbons; it has long-chain polyol esterification, etherification, acetalization and other chemical properties. . The specific gravity of this product 1.25 to 1.35 and the melting point is 212 ~ 267 ℃. It is soluble in hot water and hot dimethyl sulfoxide. Animal experiments show that polyvinyl alcohol, without stimulation, causes no significant toxicity upon subcutaneous, intramuscular, intravenous injection. Polyvinyl alcohol resin products appear as white solid with the appearance of sub-floc, granular and powder; it is non-toxic, tasteless, non-polluting and is soluble in water of 80--90 ℃. Its aqueous solution has good adhesiveness and film-forming property; it can resist most organic solvents such as oils, lubricants and hydrocarbons; it has long-chain polyol esterification, etherification, acetalization and other chemical properties. | [Uses]
It is mainly used in the textile industry, as the raw materials of warp pulp, fabric finishing agent, vinylon fiber; interior and exterior wall paint of the building, adhesives; chemical industry use it as a polymerization emulsifier, dispersant and polyvinyl formal, acetal, butyrate aldehyde resin; paper industry use it as a paper binder; agriculture use it as soil improvers, pesticide adhesion synergist and polyvinyl alcohol film; it can also be used for daily cosmetics and high-frequency quenching agent and so on.
|
| Chemical Properties | Back Directory | [Appearance]
white or cream solid | [Melting point ]
>300 °C
| [Boiling point ]
-14.5°C (rough estimate) | [bulk density]
400-670kg/m3 | [density ]
1.30 | [Tg]
73 | [Tg]
99 | [Tg]
99°C | [refractive index ]
1.3810 (estimate) | [Fp ]
79°C | [storage temp. ]
Store below +30°C. | [solubility ]
H2O: soluble (hot) | [form ]
Powder | [color ]
White to cream | [Odor]
at 100.00?%. odorless | [PH]
3.5-7.0 (40g/l, H2O, 20℃) | [Stability:]
Stable. Combustible. Dust may form explosive mixtures with air. Incompatible with strong oxidizing agents. | [Water Solubility ]
soluble in hot water | [Merck ]
14,7585 | [Dielectric constant]
1.9(Ambient) | [IARC]
3 (Vol. 19, Sup 7) 1987 | [NIST Chemistry Reference]
Polyvinyl alcohol(9002-89-5) | [EPA Substance Registry System]
Ethenol, homopolymer(9002-89-5) |
| Hazard Information | Back Directory | [Chemical Properties]
Polyvinyl alcohol occurs as an odorless, white to cream-colored granular powder. | [Definition]
ChEBI: Polyvinyl alcohol(9002-89-5) is a homopolymer macromolecule obtained by polymerisation of vinyl alcohol. It is used as a pharmaceutic aid and ophthalmic lubricant as well as in the manufacture of surface coatings artificial sponges, cosmetics, and other products.
| [Uses]
In the plastics industry in molding Compounds, surface coatings, films resistant to gasoline, textile sizes and finishing compositions; can be compounded to yield elastomers to be used in manufacture of artificial sponges, fuel hoses, etc., also in printing inks for plastics and glass, in pharmaceutical finishing, cosmetics, water-sol film and sheeting. Pharmaceutic aid (viscosity increasing agent); ophthalmic lubricant. | [Preparation]
Vinyl alcohol has not been isolated in the free state; the keto tautomer,
acetaldehyde, is much the more stable form and is always obtained:

Thus poly(vinyl alcohol) cannot be prepared from its monomer by the usual
techniques, although the polymerization of acetaldehyde with sodium
amalgam at - 80 to - 20?? has been found to give poly( vinyl alcohol) of low
molecular weight. For commercial purposes, poly(vinyl alcohol) is obtained exclusively from poly(vinyl acetate).
Poly(vinyl acetate) is readily hydrolysed by treating an alcoholic solution
with aqueous acid or alkali. Acid hydrolysis results in traces of acid in the
poly(vinyl alcohol) which are difficult to remove and which lead to instability
of the polymer; alkaline hydrolysis results in contamination of the product by
a large amount of sodium acetate which is also difficult to remove and which
has little intrinsic value. These difficulties are avoided if poly(vinyl alcohol) is
prepared from poly(vinyl acetate) by alcoholysis using a small amount of base
as catalyst. The reaction is commonly carried out by treating poly(vinyl
acetate) with methanol in the presence of sodium methoxide:

The preferred methods of preparing poly(vinyl acetate) for conversion to
poly(vinyl alcohol) are solution and suspension polymerization. The former
technique has the advantage that if polymerization is conducted in methanol
the resulting solution can be used directly without the need for isolating the
polymer; this method is the most suitable for continuous processes. Bulk
polymerized poly(vinyl acetate) tends to give low molecular weight poly(vinyl
alcohol) of poor colour.
In one continuous process, a solution of poly(vinyl acetate) in methanol
(about 20%) is mixed with the catalyst solution in a high speed in-line mixer.
The mixture then passes through a 'gelling zone' on a conveyor belt. Typically, the material is kept at 40?? for 10 minutes in this zone during which
time the alcoholysis reaction occurs; poly(vinyl alcohol) is insoluble in
methanol and a gel is produced. The gel is chopped up and neutralized with
acetic acid to stop reaction; the liquid content (whica is mainly methanol and
methyl acetate) is then expressed and recovered. The residual solid is washed
with methanol, dried and pulverized.
It is possible to control the extent to which acetate groups are replaced by
hydroxyl groups by changing the reaction conditions. In particular, the
catalyst concentration and the time of reaction have a major effect on the
degree of alcoholysis. The most common commercial types of poly(vinyl
alcohol) are the so-called partially hydrolysed grades in which 87-89% of the
acetate groups have been replaced and the completely hydrolysed grades in
which 99-100% of the acetate groups have been replaced. The degree of
alcoholysis has an effect on the properties of the polymer. | [Production Methods]
Polyvinyl alcohol is produced through the hydrolysis of polyvinyl
acetate. The repeating unit of vinyl alcohol is not used as the starting
material because it cannot be obtained in the quantities and purity
required for polymerization purposes. The hydrolysis proceeds
rapidly in methanol, ethanol, or a mixture of alcohol and methyl
acetate, using alkalis or mineral acids as catalysts. | [Brand name]
Liquifilm Tears (Allergan). | [General Description]
Polyvinyl alcohol (PVOH) is a hydrophilic linear polymer which forms copolymers of vinyl alcohol and vinyl acetate. Hence, the structural properties of polyvinyl alcohol polymers depend on the extent of polymerization and hydrolysis. Such changes cause both chemical and physical modifications such as esterification, etherification, crystallization, ion-polymer complexation in the polymer. Modified- PVOH structures are useful in biomedical applications. | [Pharmaceutical Applications]
Polyvinyl alcohol is used primarily in topical pharmaceutical and
ophthalmic formulations. It is used as a stabilizing
agent for emulsions (0.25–3.0% w/v). Polyvinyl alcohol is also used
as a viscosity-increasing agent for viscous formulations such as
ophthalmic products. It is used in artificial tears and contact lens
solutions for lubrication purposes, in sustained-release formulations
for oral administration, and in transdermal patches. Polyvinyl
alcohol may be made into microspheres when mixed with a
glutaraldehyde solution. | [Industrial uses]
Polyvinyl alcohol is a tough, whitish polymerthat can be formed into strong films, tubes, andfibers that are highly resistant to hydrocarbonsolvents. Although polyvinyl alcohol is one ofthe few water-soluble polymers, it can be renderedinsoluble in water by drawing or by theuse of cross-linking agents. | [Biochem/physiol Actions]
Poly(vinyl alcohol) (9002-89-5) is a polyhydroxy polymer, soluble in water. PVA is known to possess high mechanical strength, biocompatibility and non-toxicity. Hence, it serves as a biomedical implant material. Polymerization of vinyl acetate to poly (vinyl acetate), which is then hydrolysed to form PVA. Its application is observed in drug delivery systems, wound dressing, dialysis membranes, artificial skin, surgical repairs and cardiovascular devices.
| [Safety]
Polyvinyl alcohol is generally considered a nontoxic material. It is
nonirritant to the skin and eyes at concentrations up to 10%;
concentrations up to 7% are used in cosmetics.
Studies in rats have shown that polyvinyl alcohol 5% w/v
aqueous solution injected subcutaneously can cause anemia and
infiltrate various organs and tissues.
(mouse, oral): 14.7 g/kg
(rat, oral): >20 g/kg | [Solubility in organics]
Glycerol (hot), glycols (hot), water | [storage]
Polyvinyl alcohol is stable when stored in a tightly sealed container
in a cool, dry place. Aqueous solutions are stable in corrosionresistant
sealed containers. Preservatives may be added to the
solution if extended storage is required. Polyvinyl alcohol undergoes
slow degradation at 100°C and rapid degradation at 200°C; it
is stable on exposure to light. | [Incompatibilities]
Polyvinyl alcohol undergoes reactions typical of a compound with
secondary hydroxy groups, such as esterification. It decomposes in
strong acids, and softens or dissolves in weak acids and alkalis. It is
incompatible at high concentration with inorganic salts, especially
sulfates and phosphates; precipitation of polyvinyl alcohol 5% w/v
can be caused by phosphates. Gelling of polyvinyl alcohol solution
may occur if borax is present. | [Regulatory Status]
Included in the FDA Inactive Ingredients Database (ophthalmic
preparations and oral tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable
Non-medicinal Ingredients. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
23/24/25-36/38-39/23/24/25-68/20/21/22-20/21/22 | [Safety Statements ]
26-36/37-45-24/25 | [WGK Germany ]
1
| [RTECS ]
TR8100000
| [Autoignition Temperature]
450 °C | [TSCA ]
Yes | [HS Code ]
39053000 | [Safety Profile]
Questionable
carcinogen with experimental carcinogenic
and tumorigenic data by implant route.
Flammable when exposed to heat or flame;
can react with oxidizing materials. Slight
explosion hazard in the form of dust when
exposed to flame. To fight fire, use alcohol
foam, CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
irritating fumes. | [Hazardous Substances Data]
9002-89-5(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: > 20000 mg/kg |
|
|