| Identification | Back Directory | [Name]
ATRIAL NATRIURETIC FACTOR (1-28) (RAT) | [CAS]
88898-17-3 | [Synonyms]
RANF
RANP
ANF 1-28
ALPHA-RANP
ANP-(1-28)
RANF (1-28)
CARDIONATRIN
ANP 1-28, RAT
ANF (1-28), RAT
ANP (123-150), RAT
ANF (123-150), RAT
α-atriopeptin, rat
Atriopeptin28【rat】
Atriopeptin-28【rat】
ALPHA-ANF 1-28, RAT
ALPHA-ANP [1-28] RAT
ALPHA-ATRIOPEPTIN, RAT
ATRIAL NATRIURETIC PEPTIDE
SLRRSSCFGGRIDRIGAQSGLGCNSFRY
atrialnatriureticfactor(1-28
Atrial natriuretic factor【rat】
ANP (RAT, 1-28) (RABBIT, MOUSE)
ATRIAL NATRIURETIC PEPTIDE, RAT
M.W. 3062.43 C128H205N45O39S2
Rat atrial natriuretic factor(1-28)
TRIAL NATRIURETIC FACTOR (1-28) (RAT)
ATRIAL NATRIURETIC FACTOR (1-28) (RAT)
ATRIAL NATRIURETIC PEPTIDE (1-28), RAT
ATRIAL NATRIURETIC PEPTIDE, RAT, 100 UG
ATRIAL NATRIURETIC FACTOR (1-28) (RAT) USP/EP/BP
SLRRSSCFGGRIDRIGAQSGLGCNSFRY (DISULFIDE BRIDGE: 7-23)
A-TYPE (ATRIAL) NATRIURETIC PEPTIDE (RAT, 1-28) (RABBIT, MOUSE)
Atrial Natriuretic Factor (1-28) (Mouse, rabbit, rat)
rANF (1-28)
α-Atriopeptin, rat, α-rANP, ANF (123-150), rat, ANF 1-28, rANP
Atrial Natriuretic Peptide (ANP) (1-28), rat (Atrial natriuretic factor (1-28) (rat))
SER-LEU-ARG-ARG-SER-SER-CYS-PHE-GLY-GLY-ARG-ILE-ASP-ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS-ASN-SER-PHE-ARG-TYR
SER-LEU-ARG-ARG-SER-SER-CYS-PHE-GLY-GLY-ARG-ILE-ASP-ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS-ASN-SER-PHE-ARG-TYR RAT
H-SER-LEU-ARG-ARG-SER-SER-CYS-PHE-GLY-GLY-ARG-ILE-ASP-ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS-ASN-SER-PHE-ARG-TYR-OH
SER-SER-CYS-PHE-GLY-GLY-ARG-ILE-ASP-ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS-ASN-SER-PHE-ARG(DISULFIDE BRIDGE:CYS3-CYS18)
SER-LEU-ARG-ARG-SER-SER-(CYS-PHE-GLY-GLY-ARG-ILE-ASP-ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS)CYCLIC-ASN-SER-PHE-ARG-TYR-OH
H-SER-LEU-ARG-ARG-SER-SER-CYS-PHE-GLY-GLY-ARG-ILE-ASP-ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS-ASN-SER-PHE-ARG-TYR-OH (DISULFIDE BRIDGE: 7-23)
SER-LEU-ARG-ARG-SER-SER-CYS-PHE-GLY-GLY-ARG-ILE-ASP- ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS-ASN-SER-PHE-ARG-TYR(DISULFIDE BRIDGE:CYS7-CYS23)
SER-LEU-ARG-ARG-SER-SER-CYS-PHE-GLY-GLY-ARG-ILE-ASP-ARG-ILE-GLY-ALA-GLN-SER-GLY-LEU-GLY-CYS-ASN-SER-PHE-ARG-TYR: SLRRSSCFGGRIDRIGAQSGLGCNSFRY DISULFIDE BRIDGE CYS7-CYS23
Atrial Natriuretic Factor (1-28) (mouse, rabbit, rat) H-Ser-Leu-Arg-Arg-Ser-Ser-Cys-Phe-Gly-Gly-Arg-Ile-Asp-Arg-Ile-Gly-Ala-Gln-Ser-Gly-Leu-Gly-Cys-Asn-Ser-Phe-Arg-Tyr-OH (Disulfide bond)
(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(1R,7S,13S,16S,19S,25S,28S,31S,34S,37S,46S,49R)-49-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-hydroxy-propanoyl]amino]-4-methyl-pentanoyl]amino]-5-guanidino-pentanoyl]amino]-5-guanidi
L-Tyrosine, L-seryl-L-leucyl-L-arginyl-L-arginyl-L-seryl-L-seryl-L-cysteinyl-L-phenylalanylglycylglycyl-L-arginyl-L-isoleucyl-L-α-aspartyl-L-arginyl-L-isoleucylglycyl-L-alanyl-L-glutaminyl-L-serylglycyl-L-leucylglycyl-L-cysteinyl-L-asparaginyl-L-seryl-L-phenylalanyl-L-arginyl-, cyclic (7→23)-disulfi... | [Molecular Formula]
C128H205N45O39S2 | [MDL Number]
MFCD00076228 | [MOL File]
88898-17-3.mol | [Molecular Weight]
3062.41 |
| Questions And Answer | Back Directory | [Properties]
The Mr of human ANP is 3082 and the isoelectric point
is about 10.7. It is freely soluble in water, ethanol, and
70% acetone, and insoluble in acetone, benzene,
chloroform, and ether. ANP solution in water at
>10-4M is stable for more than a year at -20°C. | [Gene, mRNA, and precursor]
The human ANP gene (NPPA), located on chromosome
1 (1p36.22), consists of three exons, and has
AP-1, GRE, and other regulatory elements in the promoter
region. Human ANP mRNA is 855 bp long (456bp coding
sequence). Nppa first appeared in early bony fish by tandem duplication of the CNP3 gene (Nppc3). The gene
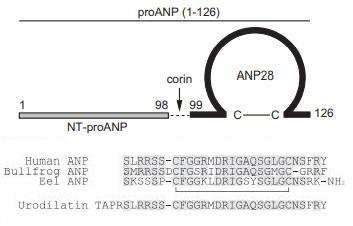
structure and its mRNA size are well conserved among teleosts, amphibians, and mammals. Human proANP1–126 is
cleaved into ANP-28 (ANP99–126) and N-terminal
(NT)-proANP1–98 by the prohormone convertase, corin. ANP mRNA is detected most abundantly in the
atrium. Some transcripts are found in the cardiac ventricle, brain, kidney, adrenal, lung, gonads, and lymphoid
tissues in mammals. In nonmammals, the heart (atrium)
is the main tissue of ANP synthesis followed by the brain,
kidney, and interrenal in frogs and teleost fish. | [Synthesis and release]
Atrial ANP synthesis and release are stimulated
principally by atrial stretch (increased blood volume) in
mammals. Corticosteroids, α-adrenergic stimulation,
and hypoxia also stimulate the gene expression as
inferred by the presence of such responsive elements in
the human gene. Increased ANP secretion in patients
with congestive and ischemic heart failure is related to
the atrial stretch and hypoxia, respectively. In eels,
ANP release is increased more profoundly by osmotic
stimulus than volemic stimulus, but the latter is a major
stimulus in trout as in mammals. The regulation of ANP
gene (Nppa) expression and release has not been examined in noncardiac tissues yet. | [Receptors]
ANP binds to the A-type natriuretic peptide receptor
(NPR-A or GC-A) with high affinity (Kd=2–3 nM). NPR-A is a single-chain receptor with an extracellular
ligand-binding domain, a membrane-spanning domain,
and intracellular guanylyl-cyclase (GC) and kinase-like
domains. The human NPR-A has 1061 aa residues with
an Mr of 118,923. NPR-A appears to exist as a tetramer,
although ANP is able to bind to a monomeric receptor.
NPR-A has been cloned in the bullfrog and eel, and a second type of NPR-A has been found in the medaka and
eel. ANP also binds to NPR-C that has only a short
intracellular domain (Kd=10 pM). The human NPR-C
consists of 540 aa residues with an Mr of 59,768. NPR-C appears to be generated by the exon shuffling of the
GC-coupled receptor. In addition, NPR-D, the second
GC-deficient receptor, has been cloned in the eel. While
NPR-C is a dimeric receptor, NPR-D exists as a tetramer,
as does NPR-A. After ANP binding, the GC domain of NPR-A is activated to catalyze the production of cGMP, which serves
as the second messenger for biological actions. NPR-C is
thought to be a clearance receptor to regulate local ANP
concentration as it exists ubiquitously in various tissues,
but the inhibition of adenylyl cyclase is suggested. | [Agonists and Antagonists]
Other cardiac NPs such as BNP and VNP are able to
bind to NPR-A with high affinities while all NPs readily
bind to NPR-C and NPR-D. C-ANF, an ANP analog with
modified intraring sequences, is a selective agonist for
NPR-C, but not for NPR-A. C-ANF administration
increases plasma ANP and enhances its biological effects.
Some NPs from snake venom, named DNP, and synthetic
chimeric NPs are used clinically as agonists. Osteocrin
containing the NP motif selectively binds to the NPR-C,
but not to the NPR-A or -B. HS-142-1 isolated from a bacterium serves as a sole
antagonist for ANP binding to NPR-A. | [Biological functions]
As expected from the secretory stimulus, ANP acts to
restore a blood volume increase to normal by decreasing
the levels of sodium and water in mammals. In addition to the vascular effect, ANP augments cardiac performance. In eels,
the effects of ANP on the brain and intestine are much
more potent and efficacious, but the effect on the kidney
is less efficacious than in mammals. ANP induces weak
antidiuresis in eels, but brisk diuresis in trout. Comparative studies in eels suggest that the fundamental action of
ANP is on sodium extrusion, but not water. | [Clinical implications]
The plasma ANP concentration is enhanced in proportion to the severity of heart failure in the New York Heart
Association (NYHA) functional classification, which
explains the use of the plasma ANP measurement for
the diagnosis of heart failure. There are innumerable
studies on the role of ANP in cardiac failure. In relation
to hypertension, significant inverse correlation is
detected between plasma ANP concentration and arterial
pressure in humans, and the administration of ANP to
hypertension patients decreases arterial pressure to a normal range. In addition, the plasma ANP concentration
increases in patients with renal failure and infectious
diseases. |
| Chemical Properties | Back Directory | [density ]
1.54±0.1 g/cm3(Predicted) | [storage temp. ]
-20°C | [solubility ]
0.05 M acetic acid: 1 mg/mL, clear, colorless
| [form ]
Solid | [color ]
White to off-white | [Water Solubility ]
Soluble to 1 mg/ml in water | [Sequence]
H-Ser-Leu-Arg-Arg-Ser-Ser-Cys-Phe-Gly-Gly-Arg-Ile-Asp-Arg-Ile-Gly-Ala-Gln-Ser-Gly-Leu-Gly-Cys-Asn-Ser-Phe-Arg-Tyr-OH(Disulfide bridge: Cys7-Cys23) |
| Hazard Information | Back Directory | [Description]
Atrial natriuretic peptide is the first cardiac hormone isolated from the atria
with potent hypotensive and natriuretic/diuretic actions. It
is a drug target for hypertension and cardiac/renal failure. The presence of a natriuretic factor in the rat heart was
first reported in 1981; it was isolated in 1983 from the rat
and human atria. | [Uses]
Atrial Natriuretic Peptide rat has been used:
- In the absorption test to confirm the specificity of the anti-NP antibodies.
- To study the effects of atrial natriuretic peptide (ANP) on renal water and sodium regulation in rats.
- To study the integrated biological effects of ANP on myocardial infarction using mice models.
| [Biochem/physiol Actions]
ANP production is regulated by factors associated with developmental, hormonal and hemodynamic processes. Its secretion is stimulated by atrial wall stretch, and ischemia. | [Clinical Use]
28-peptide, vasodilator, that increases
glomerular filtration and diuresis. | [storage]
Desiccate at -20°C | [Structure and conformation]
Human proANP consists of 126 aa residues with bioactive mature ANP at the C-terminus. Human ANP, or
ANP99–126, consists of 28 aa residues with an intramolecular ring structure of 17 aa residues, as with other NPs1. Amphibians and bony fish also possess
ANP, but birds, reptiles (except for turtles), cartilaginous
fish, and cyclostomes do not. N-terminal truncated
forms exist in the brain and an N-terminal elongated form
named urodilatin is present in the kidney. The sequence identity is low in the prosegment. The
mature ANP sequence is conserved (only one aa difference) in mammals, but is variable across different classes. Most ANPs of teleosts have an amidated
C-terminus.
 |
|
| Company Name: |
|
| Tel: |
821-50328103-801 18930552037 |
| Website: |
http://www.is0513.com/ShowSupplierProductsList13285/0.htm |
|