| Identification | Back Directory | [Name]
Carfilzomib | [CAS]
868540-17-4 | [Synonyms]
Kyprolis
Carfilzomib
CarfilzoMib salt
CarfilzoMib/PR 171
Carfilzomib, >=99%
PR-171 (CarfilzoMib)
Carfilzomib(Kyprolis)
CarfilzoMib, Free Base, >99%
(S)-4-Methyl-N-((S)-1-(((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)amino)-1-oxo
(αS)-α-[[2-(4-Morpholinyl)acetyl]amino]benzenebutanoyl-L-leucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyl-2-oxiranyl]carbonyl]butyl]-L-phenylalaninamide
(alphaS)-alpha-[(4-Morpholinylacetyl)amino]benzenebutanoyl-L-leucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-L-phenylalaninamide
L-Phenylalaninamide, (αS)-α-[[2-(4-morpholinyl)acetyl]amino]benzenebutanoyl-L-leucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyl-2-oxiranyl]carbonyl]butyl]-
N-{(2S)-2-[(Morpholin-4-ylacetyl)aMino]-4-phenylbutanoyl}-L-leucyl-N-{(2S)-4-Methyl-1-[(2R)-2-Methyloxiran-2-yl]-1-oxopentan-2-yl}-L-phenylalaninaMide
Carfilzomib (alphaS)-alpha-[(4-Morpholinylacetyl)amino]benzenebutanoyl-L-leucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-L-phenylalaninamide
(S)-4-Methyl-N-((S)-1-((S)-4-Methyl-1-((R)-2- Methyloxiran-2-yl)-1 -oxopentan-2-ylaMino)-1-oxo-3-phenylpropan-2-yl)-2-((S)-2-(2-MorpholinoacetaMido)-4-phenylbutanaMido)pentanaMide
(alphaS)-alpha-[(4-Morpholinylacetyl)amino]benzenebutanoyl-L-leucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-L-phenylalaninamide Carfilzomib(PR171) | [EINECS(EC#)]
692-054-2 | [Molecular Formula]
C40H57N5O7 | [MDL Number]
29337900 | [MOL File]
868540-17-4.mol | [Molecular Weight]
719.91 | [Description]
NCB-0846 is a novel, orally small-molecule Wnt inhibitor that inhibits TNIK (TRAF2 and NCK-Interacting Kinase). In vitro study has shown that NCM-0846 blocks Wnt signaling and exhibits remarkable anti-tumor and anti-CSC activities. In vivo study has also confirmed that NCB-0846 can inhibit Wnt-driven intestinal tumorigenesis in Apc min/+ mice and the sphere- and tumor-forming activities of colorectal cancer cells. NCB-0846 causes a dose-dependent reduction of the multiplicity and dimensions of tumors that developed in the small intestine. It also significantly suppresses the growth of the PDXs (patient-derived xenografts) established from the two patients in two more clinically relevant mouse models. Therefore, NCM-0846 has the potential to become a useful therapeutic method for tumor treatment. |
| Chemical Properties | Back Directory | [Melting point ]
204 - 208°C | [Boiling point ]
975.6±65.0 °C(Predicted) | [density ]
1.161 | [Fp ]
543.8℃ | [storage temp. ]
-20° | [solubility ]
Soluble in DMSO (up to 80 mg/ml) or in Ethanol (up to 25 mg/ml). | [form ]
solid | [pka]
13.17±0.46(Predicted) | [color ]
White | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 week. | [InChIKey]
BLMPQMFVWMYDKT-KWQOZBPPNA-N | [SMILES]
C([C@@]1(OC1)C)(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CN1CCOCC1)CCC1C=CC=CC=1)CC1C=CC=CC=1 |&1:1,6,14,18,26,r| | [CAS DataBase Reference]
868540-17-4 |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
Carfilzomib is a second-generation proteasome inhibitor that is used as a treatment in relapsed and refractory multiple myeloma. | [Definition]
ChEBI: A synthetic tetrapeptide consisting of morpholin-4-acetyl, L-2-amino-4-phenylbutanoyl, L-leucyl and L-phenylalanyl residues joined in sequence with the C-terminus connected to the amino group of (2 | [Originator]
Proteolix Inc. (United States) | [Brand name]
Kyprolis | [Clinical Use]
Carfilzomib is an irreversible inhibitor of the chymotrypsin-like protease in the proteasome and was
approved in the U.S. for the treatment of multiple myeloma. Carfilzomib was discovered by
Proteolix which was later acquired by Onyx Therapeutics who completed the development of this drug.
Carfilzomib is also undergoing clinical evaluation for additional oncology indications such as relapsed
solid tumors, lymphoma, prolymphocytic leukemia, acute myeloid leukemia and acute lymphocytic leukemia. | [Synthesis]
Carfilzomib is an analog of the natural product epoxomicin which was first synthesized in
the laboratories of Professor Crews at Yale University. Subsequent development of the SAR led to the
discovery of YU-101 in which 3 of the amino acids of this pentapeptide were modified to improve the
potency of the molecule. After licensing the molecule to Proteolix, the introduction of the morpholino
group was found to improve the solubility of the drug while maintaining efficient interaction with the
target. The most scalable route to carfilzomib closely resembles the original route developed toward
epoximicin and is described herein.
The synthesis was initiated with the amide coupling of phenyl alanine methyl ester (53) and N-Boc
leucine (54) using standard coupling reagents to afford dipeptide 55 in high yield the Scheme below. Acidic
removal of the amine protecting group followed by a second amide coupling reaction with N-Boc
homophenyl alanine provided tripeptide 56 in 85% yield for the two steps. Acidic removal of the amine
protecting group followed by acylation with chloroacetyl chloride provided β-chloro amide 57 in 67%
yield. Reaction of 57 with morpholine in the presence of catalytic amounts of potassium iodide
followed by saponification of the methyl ester with lithium hydroxide provided acid 58 in 87% yield for
the two steps. Amide coupling between acid 58 and keto-epoxyamine 59 (whose preparation is
described in the scheme below) using HOBT as the coupling reagent followed by recrystallization of the
resulting product ultimately gave carfilzomib (IX) in 75% yield.

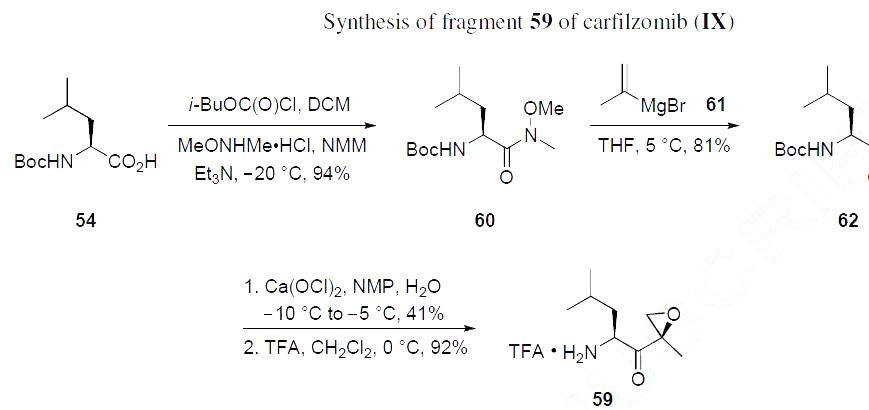
Keto-epoxyamine 59 was prepared from N-Boc leucine (54) as described in the Scheme below. Reaction of
54 with isobutyl chloroformate followed by N,O-dimethylhydroxylamine provided Weinreb amide 60 in
94% yield. Grignard addition of isopropenylmagnesium bromide 60 provided enone 62 in 81% yield.
Epoxidation of 62 with calcium hypochlorite provided a mixture of epoxides giving 41% yield of the desired isomer (presumably isolated by chromatography), and subsequent treatment with TFA liberated
the amine, providing the TFA salt of ketoepoxy amine 59 in 92% yield.
 | [target]
Proteasome | [Drug interactions]
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis. | [Metabolism]
Carfilzomib was rapidly and extensively metabolised
by mainly peptidase cleavage and epoxide hydrolysis.
Cytochrome P450 mediated mechanisms played a minor
role in overall carfilzomib metabolism. The metabolites
have no known biologic activity. | [storage]
Desiccate at -20°,unstable in solution, ready to use. | [References]
1) Bennett and Kirk (2008)?Development of proteasome inhibitors in oncology and autoimmune diseases; Curr. Opin. Drug Disc. Dev.?11?616
2) Hanada?et al.?(1992),?Epoxomicin, a new antitumor agent of microbial origin; J. Antibiot. (Tokyo),?45?174
3) Demo?et al. (2007)?Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome; Cancer Res.?67?6383
4) Kuhn?et al. (2007),?Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma; Blood,?110?328 |
| Questions And Answer | Back Directory | [description]
Carfilzomib is an irreversible proteasome inhibitor and antineoplastic agent.The mechanism of action of carfilzomib is as a Proteasome Inhibitor, Carfilzomib irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S catalytic core subunit of the proteasome, a protease complex responsible for degrading a large variety of cellular proteins. Inhibition of proteasome-mediated proteolysis results in an accumulation of polyubiquinated proteins, which may lead to cell cycle arrest, induction of apoptosis, and inhibition of tumor growth.
Carfilzomib is used in treatment of refractory multiple myeloma. Carfilzomib is associated with a low rate of serum enzyme elevations during treatment and has been implicated to rare instances of clinically apparent, acute liver injury some of which have been fatal.
Carfilzomib is a second-generation, irreversible, peptide epoxyketone class proteasome inhibitor that targets the chymotrypsin-like β5 subunit of the constitutive 20S proteasome (IC50 = 5.2 nM) and the β5i subunit of the immunoproteasome 20Si (LMP7; IC50 = 14 nM) with minimal cross reactivity to other proteases. It can induce cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, lymphoma, and various solid tumors (IC50s = 2.4-20 nM).
 | [Mechanism]
Carfilzomib is a tetrapeptide-based epoxy-proteasome inhibitor that irreversibly binds to the 20S proteasome containing the threonine N-terminal active site and the in vivo proteolysis core particle of 26S proteasome. In animal, carfilzomib has antiproliferative and apoptosis activity in vitro in solid and hematological granulocytes. In animals, carfilzomib inhibits proteasome activity in blood and tissue and delays tumor growth in multiple myeloma, hematological and solid tumor models. | [Side effects]
The most common adverse events observed in clinical trials (incidence ≥30%) were fatigue, anemia, nausea, thrombocytopenia, dyspnea, diarrhea and fever; the most common serious adverse events (overall incidence 45%) include pneumonia, acute renal failure, fever and congestive heart failure. | [Efficacy and Safety]
To assess the safety and efficacy of the drug, one study included 266 patients who had received at least two prior therapies, including bortezomib and thalidomide. It was assessed of the complete or partial disappearance of the tumor in the treated patient (overall response rate). The overall response rate was 23% and the median response time was 7.8 months.
Common adverse effects of carfilzomib were observed in more than 30% of the subjects include fatigue, low blood cell counts and platelet counts, dyspnea, diarrhea and fever. Severe adverse reactions include heart failure and dyspnea. Upon the occurrence of serious adverse reactions, the patient should be closely monitored and stop the drug treatment.
Information regarding the pharmacological effects, indications, mechanism of action, indications and side effects of Carfilzomib, a drug for treating multiple myeloma (MM), were compiled and edited by Xiao Nan of ChemicalBook. |
|
|