| Identification | Back Directory | [Name]
(R) QuinoxP(R) | [CAS]
866081-62-1 | [Synonyms]
(R) QuinoxP
(R,R)-QuinoxP*
(R) QuinoxP(R)
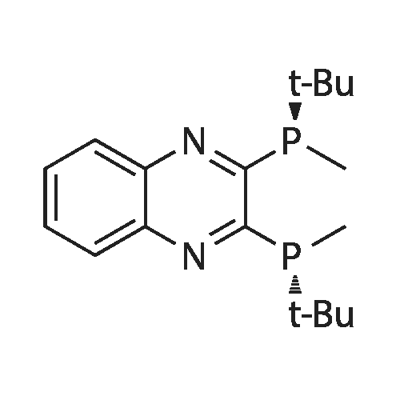
2,3-Bis((R)-tert-butyl(methyl)phosphino)quinoxaline
(R,R)-(-)-2,3-Bis(t-butylmethylphosphino)quinoxaline
(R,R)-(-)-2,3-Bis(tert-butylmethylphosphino)quinoxaline
(R,R)-(-)-2,3-Bis(tert-butylmethylphosphino)quinoxaline 97%
(R,R)-(-)-2,3-Bis(t-butylmethylphosphino)quinoxaline, min. 98% (R,R)-QuinoxP* | [Molecular Formula]
C18H28N2P2 | [MDL Number]
MFCD10565649 | [MOL File]
866081-62-1.mol | [Molecular Weight]
334.38 |
| Chemical Properties | Back Directory | [Melting point ]
100-104 °C | [alpha ]
-54.3° (c 1.0, CHCl3) | [Boiling point ]
447.6±45.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
Powder | [pka]
-0.54±0.59(Predicted) | [color ]
orange | [InChIKey]
DRZBLHZZDMCPGX-UHFFFAOYSA-N |
| Questions And Answer | Back Directory | [Reaction]
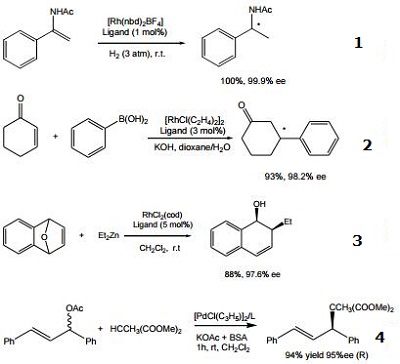
- Ligand for the rhodium-catalyzed, asymmetric hydrogenation of dehydroamino acid esters and α-enamides.
- Ligand for the rhodium-catalyzed, asymmetric 1,4-addition of arylboronic acids to α,β-unsaturated carbonyl compounds.
- Ligand for the rhodium-catalyzed, asymmetric alkylative ring opening reaction
- Ligand for the palladium-catalyzed asymmetric allylic alkylation and amination of racemic substrates.
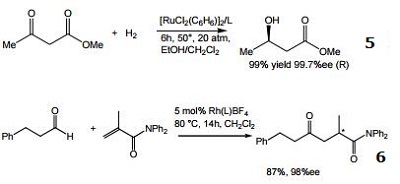
- Ligand for the ruthenium-catalyzed asymmetric hydrogenation of ketones.
- Ligand for the rhodium-catalyzed, asymmetric hydroacylation of 1,1-disubstituted alkenes with aldehydes.
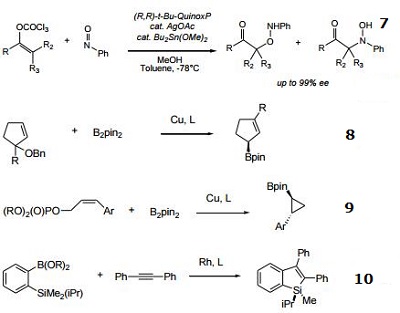
- Ligand for the silver-catalyzed asymmetric nitroso aldol reaction.
- Cu-catalyzed enantioconvergent allyllic borylation.
- Cu-catalyzed enantioselective cyclopropylation.
|
| Hazard Information | Back Directory | [Description]
(R,R)-(-)-2,3-Bis(tert-butylmethylphosphino)quinoxaline is an efficient ligand exhibiting high levels of enantiocontrol in synthetic transformations ranging from metal-catalyzed 1,4-addition of arylboronic acids to asymmetric hydrogenation to alkylative ring opening. | [Uses]
| [Application]
Air-Stable and Highly Efficient Chiral Ligands,Efficient ligand exhibiting high levels of enantiocontrol in synthetic transformations ranging from metal-catalyzed 1,4-addition of arylboronic acids to alkylative ring opening to asymmetric hydrogenation. | [General Description]
(R,R)-(-)-2,3-Bis(tert-butylmethylphosphino)quinoxaline is an air-stable C2-symmetric P-stereogenic phosphine ligand. | [Synthesis]
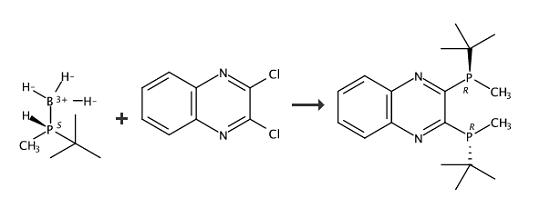
In 4 ml of tetrahydrofuran was dissolved 236 mg (2.0 mmol) of (R)-tert-butylmethylphosphine-borane (9), and the resulting solution was cooled to -78° C. with liquid nitrogen. To the cooled solution was added dropwise 1.25 ml of a 1.6 M hexane solution of n-butyllithium. Fifteen minutes later, a solution of 133 mg (0.67 mmol) of 2,3-dichloroquinoxaline (10) in 4 ml of tetrahydrofuran was added thereto dropwise while vigorously stirring to form a diphosphine-borane compound (11) as an intermediate. The liquid temperature was raised to room temperature over one hour, at which the mixture was stirred for 3 hours. One milliliter of TMEDA was added thereto, and the stirring was continued for an additional 2 hour period to complete deboranation. The reaction was ceased by addition of 1M hydrochloric acid. The reaction mixture was extracted with hexane. The extract was washed successively with 1M hydrochloric acid and a saturated sodium chloride aqueous solution and dried over sodium sulfate. The solvent was removed by evacuation, and the residue was purified by silica gel column chromatography (mobile phase: hexane/ethyl acetate=30/1) to give (R,R)-2,3-bis(tert-butylmethylphosphino)quinoxaline (12) as an orange solid. Recrystallization from 1.7 ml of hot methanol gave orange crystals (>99% ee) in a yield of 80%. |
|
| Company Name: |
ChemicalRIM Co., Ltd Gold
|
| Tel: |
15184345951 |
| Website: |
http://www.is0513.com/ShowSupplierProductsList988301/0.htm |
|