| Identification | Back Directory | [Name]
(R)-1-{(S)-2-[DI(1-NAPHTHYL)PHOSPHINO]FERROCENYL}ETHYLDI(3,5-XYLYL)PHOSPHINE | [CAS]
851308-40-2 | [Synonyms]
(R)-(-)-1-[(S)-2-(Di-1-naphthylphosphino)ferrocenyl]eth
(R)-1-[(S)-2-(Di-1-naphtylphosphino)ferrocenyl]-ethyl-di-3,5-xylylphosphine
(R)-1-{(SP)-2-[Di(1-naphthyl)phosphino]ferrocenyl}ethyldi(3,5-xylyl)phosphine
(R)-1-{(SP)-2-[Di(1-naphthyl)phosphino]ferrocenyl}ethyldi(3,5-xylyl)phosphine >=97%
(R)-(-)-1-[(S)-2-(Di-1-naphthylphosphino)ferrocenyl]ethyldi-3,5-xylylphosphine,Min.97%
(R)-(-)-1-[(S)-2-(Di-1-naphthylphosphino) ferrocenyl]ethyldi-3,5-xylylphosphine, min. 97%
(r,r)-1-{1-[bis(3,5-dimethylphenyl)phosphino]ethyl}-2-[di(1-naphthyl)phosphino]ferrocene (acc to cas) | [Molecular Formula]
C43H39P2.C5H5.Fe | [MDL Number]
MFCD08561162 | [MOL File]
851308-40-2.mol | [Molecular Weight]
738.669 |
| Questions And Answer | Back Directory | [Reactions]
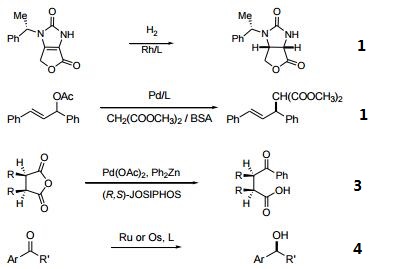
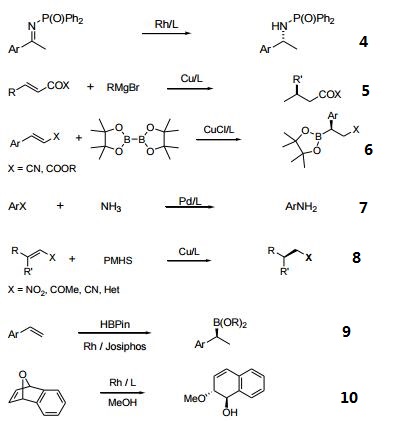
- Ferrocenylphosphine ligands of the type cpFecp(PR2)(*CH(CH3)PR'2) are a class of asymmetric ligands developed at Solvias in Basel, Switzerland. Ligands of this type are currently used industrially in the stereoselective synthesis of commercial products. A unique feature of these bidentate ligands is the presence of a fixed phosphine moiety and a stereogenic, functionalized side chain, which can be easily modified to accommodate electronic and steric requirements. Based on a versatile synthetic procedure starting with optically active ferrocenes of the type cpFecp(PR2)(*CH(CH3)X) [X = OAc or NR2], a variety of donor atoms can be introduced into the side chain.4 These ferrocene based phosphine ligands have wide application in the stereoselective hydrogenation of substituted acetamidoacrylates, enol acetates, β-ketoesters and simple alkenes.
- Useful as a ligand in Pd-catalyzed C-N bond-forming reactions.
- Pd-catalyzed enantioselective alkylative desymmetrization of meso-succinic anhydrides.
- Asymmetric hydrogenation of ketones and phosphinylketimines.
- Michael addition of Grignard reagents to α,α-unsaturated esters and thioesters.
- Boration of α,α-unsaturated esters and nitriles.
- Reaction of aryl halides with ammonia.
- Cu-catalyzed reduction of activated C=C bonds with PMHS.
- Regio- and enantioselective hydroboration of vinyl arenes.
- Rh-catalyzed asymmetric ring-opening reactions of oxabicyclic alkenes.
|
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|