| Identification | Back Directory | [Name]
2-HYDROXY-1,3,5-BENZENETRICARBALDEHYDE | [CAS]
81502-74-1 | [Synonyms]
1,3,5-Benzenetricarboxaldehyd
2-HYDROXY-1,3,5-BENZENETRICARBALDEHYDE
2-Hydroxybenzene-1,3,5-tricarbaldehyde
2-HYDROXYBENZENE-1,3,5-TRICARBOXALDEHYDE
2-Hydroxy-1,3,5-benzenetricarboxaldehyde
1,3,5-Benzenetricarboxaldehyde,2-hydroxy-||| | [Molecular Formula]
C9H6O4 | [MDL Number]
MFCD01314207 | [MOL File]
81502-74-1.mol | [Molecular Weight]
178.14 |
| Chemical Properties | Back Directory | [Melting point ]
179 °C | [Boiling point ]
282.9±40.0 °C(Predicted) | [density ]
1.449±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
powder to crystal | [pka]
4.20±0.23(Predicted) | [color ]
White to Yellow | [λmax]
335nm(EtOH)(lit.) |
| Hazard Information | Back Directory | [Chemical Properties]
2-Hydroxy- 1,3,5-benzenetricarbaldehyde has a very limited solubility in
most organic solvents and in water. It dissolves well in DMSO and in hot DMF,
and the latter can be used as a recrystallization solvent. While the trialdehyde 3 is
colorless in its free acid form, its anion absorbs light in the visible region giving
the compound a yellow coloration in solutions when dissociation can occur. The
yellow sodium salt of 2-hydroxy- 1,3,5-benzenetricarbaldehyde is only slightly
soluble in water. | [Synthesis]
2-Hydroxybenzene-1,3,5-tricarbaldehyde is synthesized from phenol or 4-hydroxybenzaldehyde[1].
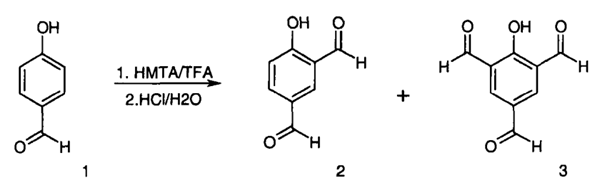
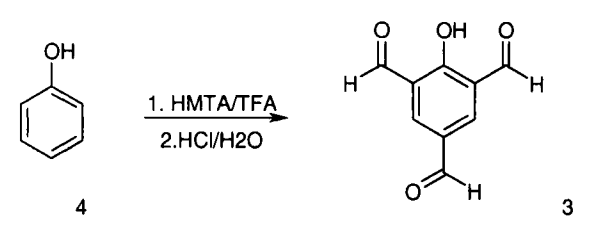 | [References]
[1] A. ANDERSON. A Convenient One-Step Synthesis of 2-Hydroxy-1,3,5-Benzenetricarbaldehyde[J]. Synthetic Communications, 2000. DOI:10.1080/00397910008086933. |
|
|