| Identification | Back Directory | [Name]
kijanimicin | [CAS]
78798-08-0 | [Synonyms]
Sch 25663
kijanimicin
Kijanimicin sodium salt
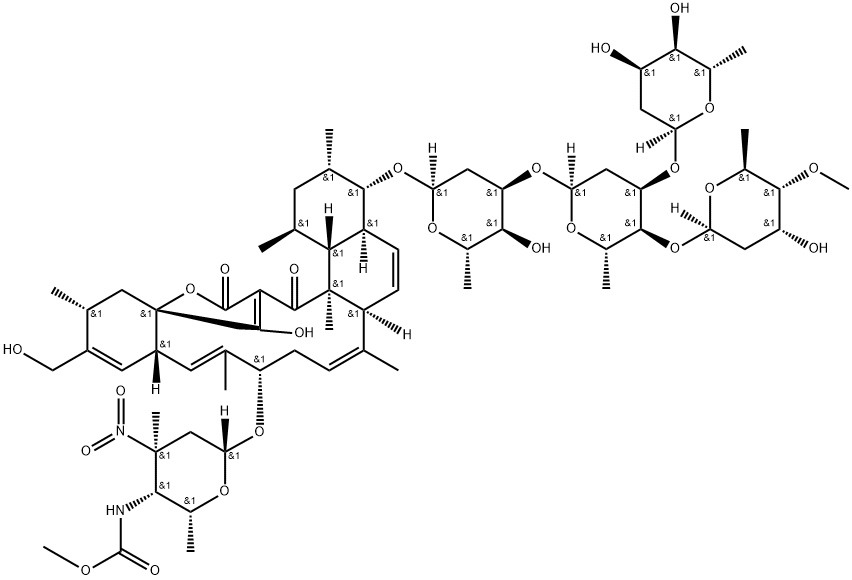
18H-16a,19-Metheno-16aH-benzo[b]naphth[2,1-j]oxacyclotetradecin-18,20(1H)-dione, 4-[(O-2,6-dideoxy-α-L-ribo-hexopyranosyl-(1→3)-O-[2,6-dideoxy-4-O-methyl-β-L-ribo-hexopyranosyl-(1→4)]-O-2,6-dideoxy-α-L-ribo-hexopyranosyl-(1→3)-2,6-dideoxy-α-L-ribo-hexopyranosyl)oxy]-2,3,4,4a,6a,9,10,12a,15,16,20a,20... | [Molecular Formula]
C67H100N2O24 | [MDL Number]
MFCD01939675 | [MOL File]
78798-08-0.mol | [Molecular Weight]
1317.52 |
| Chemical Properties | Back Directory | [density ]
1.35±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMF: soluble; DMSO: soluble; Ethanol: soluble; Methanol: soluble | [form ]
A solid | [pka]
4.50±1.00(Predicted) |
| Hazard Information | Back Directory | [Uses]
Kijanimicin is a tetronic acid related to saccharocarcin, chlorothricin, versipelostatin and tetrocarcin. Like the tetrocarcins, kijanimicin contains an unusual nitroaminoglycoside. Kijanimicin is a potent antibacterial, antimalarial and antitumour active. Several members of this class have received considerable literature focus. Versipelostatin inhibits transcription from the promoter of GRP78, a gene that is activated as part of a stress signalling pathway under glucose deprivation resulting in unfolded protein response (UPR). The UPR-inhibitory action is seen only in conditions of glucose deprivation and causes selective and massive killing of the glucose-deprived cells. Tetrocarcin A appears to target the phosphatidylinositide-3'-kinase/Akt signalling pathway. | [Uses]
Kijanimicin is an antibacterial tetronic acid compound. | [storage]
Store at -20°C |
|
| Company Name: |
Lynnchem
|
| Tel: |
86-(0)29-85992781 17792393971 |
| Website: |
http://www.lynnchem.com/ |
| Company Name: |
Novachemistry
|
| Tel: |
44-20819178-90 02081917890 |
| Website: |
https://www.novachemistry.com/ |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|