| Identification | Back Directory | [Name]
16alpha,17,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione 21-acetate | [CAS]
77017-20-0 | [Synonyms]
BNKY015-BD01
Budesonide Impurity 12
Budesonide Impurity 33
Pregna-1,4,9(11)-triene-16,17-diol-3,20-dione 21-acetate
16,17-Dihydroxypregna-1,4,9(11)-triene-3,20-dione 21-acetate
21-(Acetyloxy)-16α,17-dihydroxypregna-1,4,9(11)-triene-3,20-dione
16α,17α,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione-21-acetate
16alpha,17,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione 21-acetate
(16α)-21-Acetyloxy-16,17-dihydroxy-pregna-1,4,9(11)-triene-3,20-dione
16-alpha,17-alpha-dihydroxy-3,20-dioxopregna- 1,4,9(11)-trien-21-yl acetate
Pregna-1,4,9(11)-triene-3,20-dione, 21-(acetyloxy)-16,17-dihydroxy-, (16α)-
Pregna-1,4,9(11)-triene-3,20-dione,21-(acetyloxy)-16,17-dihydroxy-, (16a)- (9CI) | [EINECS(EC#)]
278-593-2 | [Molecular Formula]
C23H28O6 | [MDL Number]
MFCD12031469 | [MOL File]
77017-20-0.mol | [Molecular Weight]
400.47 |
| Chemical Properties | Back Directory | [Melting point ]
213-215 °C(Solv: acetone (67-64-1); ligroine (8032-32-4)) | [Boiling point ]
576.7±50.0 °C(Predicted) | [density ]
1.31±0.1 g/cm3(Predicted) | [solubility ]
soluble in Acetone, Dichloromethane | [pka]
11.92±0.70(Predicted) | [Water Solubility ]
38.72mg/L at 25℃ | [LogP]
2.26 at 25℃ |
| Hazard Information | Back Directory | [Uses]
Intermediate in the preparation of antiinflammatory compounds. | [Synthesis]
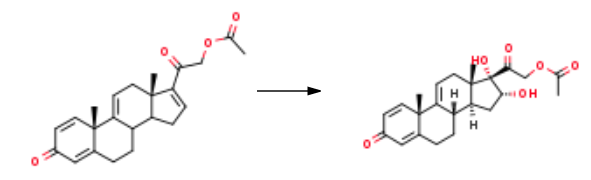
2-((10S,13S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate (1 kg, 2.73 mol), acetone (50 L) and purified water (2.6 L) were added to the reaction vessel, and dissolved by stirring at room temperature; Formic acid (0.4 L, 10.60 mol) and potassium permanganate (1.2 kg, 7.59 mol) were added in that order, and the reaction was stirred at room temperature for 1 h. The reaction was quenched by the addition of saturated sodium hydrogen sulfite solution (10 L) and manganese dioxide, and some solid impurities were removed by suction filtration, and the filtrate was evaporated under reduced pressure to give a large white solid. The filter cake was washed with water to give 16alpha,17,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione 21-acetate (1.01 kg, 92.2%).
 |
|
|