| Identification | Back Directory | [Name]
4,5-DICHLORO-1,2,3-DITHIAZOLIUM CHLORIDE | [CAS]
75318-43-3 | [Synonyms]
D14145
Appel salt
Appel's Salt
4,5-Dichlorodithiaz
4,5-dichlorodithiazol-1-ium
4,5-DICHLORO-1,2,3-DITHIAZOLIUM
4,5-dichlorodithiazol-1-ium chloride
4,5-Dichlorodithiazol-2-ium chloride
4,5-Dichloro-[1,2,3]dithiazol-2-ylium
4,5-Dichloro-1,2,3-dithiazolium chloride
4,5-dichloro-3H-dithiazol-3-ium chloride
4,5-dichloro-5H-dithiazol-3-ium chloride
DICHLORO-1,2,3-DITHIAZOL-1-YLIUM CHLORIDE
4,5-Dichloro-1,2,3-dithiazol-1-ium chloride
4,5-Dichloro-1,2,3-dithiazol-2-ylium chloride
dichloro-1lambda4,2,3-dithiazol-1-ylium chloride
4,5-Dichloro-1,2,3-dithiazol-2-ylium chloride, tech | [Molecular Formula]
C2Cl3NS2 | [MDL Number]
MFCD03426194 | [MOL File]
75318-43-3.mol | [Molecular Weight]
208.5 |
| Hazard Information | Back Directory | [Description]
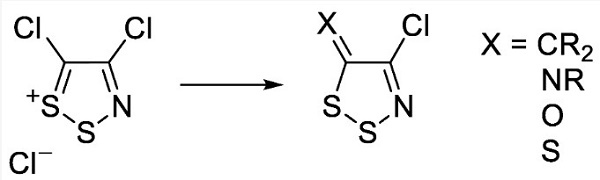
4,5-Dichloro-1,2,3-dithiazolium chloride is an useful organosulfur compound. It is the chloride salt of the 4,5-dichloro-1,2,3-dithiazolium cation. It is a green solid that is poorly soluble in organic solvents. Most primary arylamines react readily with 4,5-dichloro-1,2,3-dithiazolium chloride to give, after treatment with tertiary amine base (2 equiv.), the corresponding N-aryl-4-chloro-5H-1,2,3-dithiazolimines in good to excellent yields. This compound is commonly used to synthesize monocyclic 1,2,3-dithiazoles which act as fungicides, antibacterials, antivirals or anticancer agents[1-2].
| [Uses]
Synthon in heterocyclic chemistry; in conversion of alcohols and carboxylic acids to esters. | [References]
[1] Kalogirou A, et al. The Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with Sulfimides: A New Synthesis of N-Aryl-1,2,3-dithiazolimines. Molecules, 2009; 14: 2356–2362.
[2] Plakas K, et al. Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with 2-(Phenylsulfonyl)acetonitrile. Molbank, 2022; 2022: M1322. |
|
|