| Identification | Back Directory | [Name]
2,3-NAPHTHALENEDICARBOXYLIC ANHYDRIDE | [CAS]
716-39-2 | [Synonyms]
EA253
2,3-NAPHTHALI
2,3-NAPHTHALIC ANHYDRIDE
naphtho[2,3-c]furan-1,3-dione
benzo[f][2]benzofuran-1,3-dione
benzo[f]isobenzofuran-1,3-quinone
2,3-NAPHTHALENEDICARBOXYLIC ANHYDRIDE
2,3-NaphthalenedicarboxylicAnhydride>
1,3-Dihydronaphtho[2,3-c]furan-1,3-dione
2,3,NAPHTHALENEDICARBOXYLIC ACIDANHYDRIDE
2,3-NAPHTHALENEDICARBOXYLIC ANHYDRIDE ISO 9001:2015 REACH | [EINECS(EC#)]
211-936-6 | [Molecular Formula]
C12H6O3 | [MDL Number]
MFCD00059091 | [MOL File]
716-39-2.mol | [Molecular Weight]
198.17 |
| Chemical Properties | Back Directory | [Melting point ]
246 °C | [Boiling point ]
275-280 °C(Press: 100 Torr) | [density ]
1.449±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly) | [form ]
Solid | [color ]
Pale Beige | [Stability:]
Moisture Sensitive | [InChI]
InChI=1S/C12H6O3/c13-11-9-5-7-3-1-2-4-8(7)6-10(9)12(14)15-11/h1-6H | [InChIKey]
IZJDCINIYIMFGX-UHFFFAOYSA-N | [SMILES]
O1C(=O)C2=CC3C(C=C2C1=O)=CC=CC=3 | [EPA Substance Registry System]
Naphtho[2,3-c]furan-1,3-dione (716-39-2) |
| Hazard Information | Back Directory | [Description]
A few years ago, the product was still synthesized from the corresponding diacid, but in recent years, it has been difficult to find the shadow of the diacid in the market, and the sales of this product have been repeatedly restricted, resulting in high prices. | [Uses]
2,3-Naphthalic anhydride is used as a reagent to synthesize analogues of Thalidomide (T338850), an inhibitor of tumour necrosis factor that was once abandoned because it caused birth defects, but is currently used as an inhibitor of angiogenesis in patients with multiple myeloma. | [Preparation]
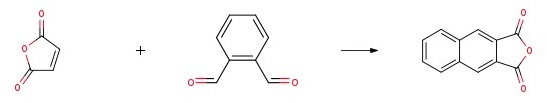
Add anhydrous tetrahydrofuran (its mass ratio to maleic anhydride is 1.5:1), cat-1 (0.05 mole), maleic anhydride (1 mole), and o-phthalaldehyde (1.5 mole) to the reactor. After adding triethylamine (5 moles), adjust the temperature below -40°C. Silicon tetrachloride (4 moles) was added dropwise to the reaction system and the reaction was placed at -40 °C for 4 h. The mixture was heated to reflux for 20 h. After the end of the reaction, concentrating the solution to a certain concentration with diatomaceous earth filtration.
A light yellow solid, namely 2,3-Naphthalenedicarboxylic Anhydride, is obtained by precipitation, filtration, recrystallization and drying. Its yield reaches 88%.
 |
|
|