904326-93-8
中文名稱
6-(4-嗎啉基)-3-吡啶硼酸
英文名稱
6-(4-morpholinyl)-3-pyridinylboronic acid
CAS
904326-93-8
分子式
C9H13BN2O3
分子量
208.02
MOL 文件
904326-93-8.mol
更新日期
2025/01/15 10:23:50
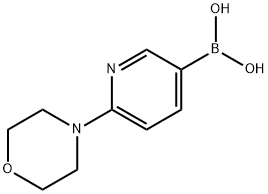 904326-93-8 結(jié)構(gòu)式
904326-93-8 結(jié)構(gòu)式
基本信息
中文別名
2-嗎啉基-5-吡啶硼酸6-嗎啉基吡啶-3-硼酸
(6-嗎啉代吡啶-3-基)硼酸
6-(4-嗎啡啉)-3-吡啶硼酸
6-(4-嗎啉基)-3-吡啶硼酸
2-(嗎啡啉-1-基)吡啶-5-硼酸
6-(4-嗎啉基)-3-吡啶硼酸 1G
6-(嗎啉基)吡啶-3-硼酸 (含有數(shù)量不等的酸酐)
英文別名
Methylpyridine-4-boronic acid2-MORPHOLINOPYRIDINE-5-BORONIC ACID
2-MORPHOLINO-5-PYRIDINEBORONIC ACID
6-MORPHOLINOPYRIDINE-3-BORONIC ACID
6-Morpholino-3-pyridineboronic Acid
6-morpholinopyridin-3-ylboronic acid
6-morpholin-4-yl-pyridineboronic acid
6-morpholinopyridin-3-yl-3-boronic acid
6-(4-Morpholino)pyridine-3-boronic acid
6-(4-MORPHOLINYL)-3-PYRIDINEBORONIC ACID
物理化學(xué)性質(zhì)
沸點(diǎn)458.6±55.0 °C(Predicted)
密度1.30±0.1 g/cm3(Predicted)
儲(chǔ)存條件under inert gas (nitrogen or Argon) at 2-8°C
酸度系數(shù)(pKa)4.68±0.18(Predicted)
形態(tài)粉末晶體
顏色白色至橙色再至綠色
CAS 數(shù)據(jù)庫904326-93-8
