| Identification | Back Directory | [Name]
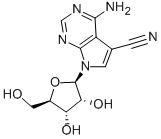
4-AMINO-5-CYANO-7-(BETA-D-RIBOFURANOSYL)PYRROLO[2,3-D]PYRIMIDINE | [CAS]
606-58-6 | [Synonyms]
e-212
e-212-1
a-399-y4
NSC 63701
NSC 99843
siromycin
uramycinb
vengicide
unamycin-b
TOYOCAMYCIN
naritheracin
Neuro 000027
antibiotice212
ahygroscopin-b
antibiotic1037
cyanotubericidin
antibiotica-399-y4
toyocamycinnucleoside
TOYOCAMYCIN (VENGICIDE)
7-Deaza-7-cyanoadenosine
7-CYANO-7-DEZXAADENOSINE
4-amino-5-cyano-7-(d-ribofuranosyl)-7h-pyrrolo(2,3-d)pyrimidine
4-AMINO-5-CYANO-7-(BETA-D-RIBOFURANOSYL)PYRROLO[2,3-D]PYRIMIDINE
4-Amino-5-cyano-7-(beta-D-ribofuranosyl)-7H-pyrrolo[2.3-d]pyrimidine
4-Aminopyrrolo[2,3-d]pyrimidine-5-carbonitrile 7-(β-D-ribofuranoside)
4-Amino-7-β-D-ribofuranosyl-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile
4-AMINO-7-SS-D-RIBOFURANOSYL-7H-PYRROLO[2,3-D]PYRIMIDINE-5-CARBONITRILE
7H-Pyrrolo[2,3-d]pyrimidine-5-carbonitrile, 4-amino-7-β-D-ribofuranosyl-
3-d)pyrimidine-5-carbonitrile,4-amino-7-beta-d-ribofuranosyl-7h-pyrrolo(
4-amino-7-beta-d-ribofuranosyl-7h-pyrrolo(2,3-d)pyrimidine-5-carbonitrile
Cyanotubercidin, UnaMycin B, Vengicide, E 212, 1037, E 212, Anhygroscopin B, Naritheracin | [Molecular Formula]
C12H13N5O4 | [MDL Number]
MFCD00866513 | [MOL File]
606-58-6.mol | [Molecular Weight]
291.26 |
| Chemical Properties | Back Directory | [Melting point ]
243°; mp 239-243° | [alpha ]
D16 -45.7° (c = 1.05 in 0.1N HCl) | [Boiling point ]
433.28°C (rough estimate) | [density ]
1.3067 (rough estimate) | [refractive index ]
1.7000 (estimate) | [storage temp. ]
2-8°C | [solubility ]
DMSO: soluble0.90 - 1.10mg/mL, clear, colorless | [form ]
solid | [pka]
12.31±0.70(Predicted) | [color ]
White or off-white | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| Hazard Information | Back Directory | [Description]
Toyocamycin (606-58-6) is an adenosine analog which inhibits ribozyme self cleavage in mammalian cells, EC50?= 0.4 μM (for expression of a luciferase reporter).1?A potent inhibitor of ER stress-induced XBP1 mRNA splicing.2?It suppresses thapsigargin-, tunicamycin- and 2-deoxyglucose-induced XBP1 mRNA splicing in HeLa cells without affecting ATF6 and PERK activation. Although unable to inhibit IRE1α phosphorylation, toyocamycin prevented IRE1α-induced XBP1 mRNA cleavage in vitro.?It inhibits not only ER stress-induced but also constitutive activation of XBP1 expression in multiple myeloma cell lines as well as in primary patient samples.2?Displays synergistic effects with bortezomib. Toyocamycin inhibits unfolded protein response and induces apoptosis in pancreatic cancer cells.3 | [Uses]
Toyocamycin is a natural adenosine analog first isolated from Streptomyces and shown in early studies to be cytotoxic to bacteria, fungi, and cancer cells and to have antiviral activities. Toyocamycin prevents IRE1α-induced mRNA cleavage (IC50 = 80 nM) and inhibits constitutive activation of XBP1 in multiple myeloma cell lines. It is used to study IRE1α action in the endoplasmic reticulum stress response, particularly in the context of cancer. It also inhibits phosphatidylinositol kinase in vitro (IC50 = 3.3 μg/ml), but not in cells, and blocks the ribosomal RNA-processing kinase Rio1 (IC50 = ~30 nM).[Cayman Chemical] | [Uses]
Toyocamycin is a pyrrolopyrimidine nucleoside isolated from Streptomyces toyocaensis in 1956. Toyocamycin, like other members of pyrrolopyrimidine class, is an adenosine nucleotide antimetabolite, with a broad spectrum of action against bacteria, fungi, protozoans and mammalian cell lines. | [Definition]
ChEBI:Toyocamycin is an N-glycosylpyrrolopyrimidine that is tubercidin in which the hydrogen at position 5 of the pyrrolopyrimidine moiety has been replaced by a cyano group. It has a role as an antimetabolite, an antineoplastic agent, a bacterial metabolite and an apoptosis inducer. It is a N-glycosylpyrrolopyrimidine, a nitrile, a ribonucleoside and an antibiotic antifungal agent. | [Biochem/physiol Actions]
Studies have implicated that toyocamycin blocks the replication of fowl plague virus. | [storage]
Store at -20°C | [References]
1) Yen?et al. (2006),?Identification of inhibitors of ribozyme self-cleavage in mammalian cells via high-throughput screening of chemical libraries; RNA,?12?797
2) Ri?et al.?(2012),?Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing; Blood Cancer J.,?2?e79
3) Chien?et al.?(2014),?Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells; Oncotarget,?5?4881 |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|