| Identification | Back Directory | [Name]
guanethidine | [CAS]
55-65-2 | [Synonyms]
Dopom
C07036
Eutensol
Abapresin
Octatensine
guanethidine
guanethidine USP/EP/BP
2-[2-(azocan-1-yl)ethyl]guanidine
1-(2-perhydroazocin-1-ylethyl)guanidine
N-[2-(Octahydroazocin-1-yl)ethyl]guanidine
Guanidine, N-[2-(hexahydro-1(2H)-azocinyl)ethyl]- | [EINECS(EC#)]
200-241-3 | [Molecular Formula]
C10H22N4 | [MDL Number]
MFCD00242835 | [MOL File]
55-65-2.mol | [Molecular Weight]
198.308 |
| Chemical Properties | Back Directory | [Boiling point ]
325.64°C (rough estimate) | [density ]
0.9402 (rough estimate) | [refractive index ]
1.4910 (estimate) | [storage temp. ]
Store at -20°C | [pka]
pKa 11.4 (Uncertain) |
| Hazard Information | Back Directory | [Description]
Guanethidine is used for severe hypertension when the use of the more generally
accepted drugs turns out to be unsuccessful. It is a powerful, long-lasting antihypertensive
drug; however, it affects a patient’s blood pressure only in the orthostatic position, and not
when lying down.
Guanethidine is a very powerful and long-lasting drug, and its action often lasts 2–3
days after its use has been stopped. | [Originator]
Ismelin,Ciba,US,1960 | [Uses]
Antihypertensive. | [Definition]
ChEBI: A member of the class of guanidines in which one of the hydrogens of the amino group has been replaced by a 2-azocan-1-ylethyl group. | [Manufacturing Process]
13.6 grams of chloroacetyl guanide is added while stirring to a solution of
22.6 grams of heptamethylene imine in 200 ml of benzene. After warming for
1 hour, and then cooling, the solution is filtered and the filtrate concentrated
under reduced pressure. The residue, containing the 2-(1-N,N-heptamethylene-imino)-aceticacid guanide, is suspended in tetrahydrofuran
and added to a refluxing solution of 6 grams of lithium aluminum hydride in
tetrahydrofuran. After completion of the reaction, the excess of lithium
aluminum hydride is decomposed by adding water, then aqueous sodium
hydroxide. The solid material is filtered off, the filtrate is acidified with sulfuric
acid and the 2-(1-N,N-heptamethylene-imino)-ethyl-guanidine sulfate can be
recovered and recrystallized from aqueous ethanol, MP 276° to 281°C (with
decomposition). | [Brand name]
Ismelin (Novartis). | [Therapeutic Function]
Antihypertensive | [Biological Functions]
Guanethidine (Ismelin) is a powerful antihypertensive
agent that is quite effective in the treatment of moderate
to severe hypertension. It is most frequently used in
the treatment of severe hypertension that is resistant to
other agents.
Guanethidine exerts its effects at peripheral sympathetic
nerve endings following its active transport into the
nerve varicosities by the neuronal amine transport system.
This is the same uptake system that transports norepinephrine
into the varicosity).The accumulation
of guanethidine in adrenergic neurons,
through an as yet unexplained mechanism, disrupts the
process by which action potentials trigger the release of
stored norepinephrine and other cotransmitters from
nerve terminals. It is this action of guanethidine that is
primarily responsible for its antihypertensive properties.
Parasympathetic function is not altered, a fact that
distinguishes guanethidine from the ganglionic blocking
agent.
Guanethidine is suitable for oral use, and this is its
usual route of administration. However, absorption
from the gastrointestinal tract is variable. The half-life
of guanethidine is 5 days, with about one-seventh of the
total administered dose eliminated per day. The slow
elimination contributes to the cumulative and prolonged
effects of the drug.
Guanethidine reduces blood pressure by its ability
to diminish vascular tone; both the arterial and venous
sides of the circulatory system are involved. The resulting
venous pooling contributes to orthostatic hypotension,
a prominent feature of guanethidine treatment.
The reduction in blood pressure is more prominent
when the patient is standing than recumbent. | [General Description]
Guanethidine or guanethidinesulfate [C10H22N4·H2SO4]. | [General Description]
Guanethidinehas been classified traditionally as an adrenergic blockingagent because it can prevent the release of norepinephrinefrom postganglionic neurons in response to adrenergic stimulation.Guanethidine and other compounds discussed in thissection have other actions on catecholamine metabolism andcan cause significant depletion of these amines in adrenergicneurons. They do not interfere with release of epinephrinefrom the adrenal medulla. | [Mechanism of action]
Unlike adrenoblockers, guanethidine does not act on effector cells. It acts on branched
ends of sympathetic peripheral nerve fibers and permeates into the neuron by the same
mechanism of reverse uptake that returns norepinephrine from the synaptic cleft to neu�ron endings. Inside the neuron, guanethidine accumulates and competes with norepi�nephrine for storage space as granules. With an increase in guanethidine concentration,
norepinephrine is replaced and thus the quantity of neurotransmitters capable of being
released is reduced. In response to stimulation, the nerve may release guanethidine,
which, however, is not an adrenergic receptor stimulant. In addition to this disturbance
and the presence of stores of catecholamines in adrenergic nerve endings, guanethidine
also acts on the stores of catecholamines in organs such as the heart, spleen, and aorta.Since it does not pass through the blood–brain barrier, it does not act on the central sym�pathetic neurons. | [Side effects]
Guanethidine may aggravate congestive heart failure
or actually precipitate failure in patients with marginal
cardiac reserve, owing to its ability to produce vascular
volume expansion, edema, and a reduced
effectiveness of sympathetic cardiac stimulation.
Guanethidine is contraindicated in patients with
pheochromocytoma because the drug may release catecholamines
from the tumor. The concomitant use of
monoamine oxidase (MAO) inhibitors and guanethidine
is also to be avoided, since this combined drug
treatment eliminates two of the principal mechanisms
for terminating the actions of the catecholamines and
certain other adrenomimetic drugs, that is, biotransformation
and neuronal uptake. Dangerously high concentrations
of catecholamines at receptor sites are possible.
| [Synthesis]
Guanethidine, |?-(1-azacyclooctyl)ethylguanidine (12.3.4), is synthesized
in the following straightforward manner. Azocine is alkylated by chloracetonitrile, which
forms 1-azocinylacetonitrile (12.3.2), which is reduced by lithium aluminum hydride into
1-(2-aminoethyl)azocine (12.3.3). Reacting this with S-methylthiourea gives guanethidine
(12.3.4) [77¨C79].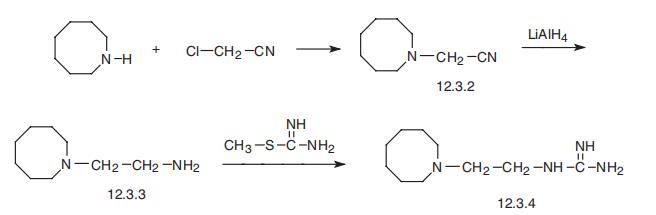
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Website: |
www.leancare.co.uk |
|