| Identification | Back Directory | [Name]
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropyridin-2(1H)-one | [CAS]
545445-44-1 | [Synonyms]
in-4-yL
Apixaban-1
HSK00001094
Apixaban Impurity H
Apixaban Impurity P
RSYY(Apremilast)-36
Apixaban Intermediates
Apixaba inretMediate B
Apixaban Intermediate B
Apixaban Intermediate 2
Impurity of apixaban383
-1-[4-(2-oxopiperidin-1-yL
Apixaban Dihydro pyridenone
]-5,6-dihydro-1H-pyridin-2-one
ApixabanGF Impurity 1 ABCDEFGHJKL
APIXABAN CAS 503612-47-3 INTERMEDIATES
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)
2(1H)-Quinolinone,3,4-dihydro-13-hydroxy-
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)
5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phen...
5,6-Dihydro-3-Morpholino-1-(4-(2-oxo Piperidin-1-yl) Ph)Py)2(1H) one
3-Morpholino-1-[4-(2-oxo-1-piperidyl)phenyl]-5,6-dhydropyridin-2(1H)-one
5,6-Dihydro-3-morpholino-1-(4-(2-oxopiperidin-1-phenyl)pyridin-2(1H)-one
3-Morpholino-1-[4-(2-oxo-1-piperidyl)phenyl]-5,6-dihydropyridin-2(1H)-one
5-morpholin-4-yl-1-[4-(2-oxopiperidin-1-yl)phenyl]-2,3-dihydropyridin-6-one
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropyridin-2(1H)-one
5,6-Dihydro-3-(morpholinyl)-1[4-(2-oxo-1-
piperidinyl)phenyl]-2(1H)-pyridinone
5,6-dihydro-3-(4-morpholine)-1-[4-(2-oxy-1-piperidyl) phenyl ]-2(1 H)-pyridone
3-Morpholin-4-yl-1-[4-(2-oxopiperidin-1-yl)phenyl]-5,6-dihydro-1H-pyridin-2-one
5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phenyl]-2(1H)-pyridinone
3-(4-Morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phenyl]-5,6-dihydro-2(1H)-pyridinone
5, 6-dihydro-3 -(4-morpholine)-1-[4-(2-oxo-1-piperidyl) phenyl]-2(1H) -pyridinone
2(1H)-Pyridinone, 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phenyl]-
3-Morpholin-4-yl-1-[4-(2-oxopiperidin-1-yl)phenyl]-5,6-dihydro-1H-pyridin-2-one
(Apixaban)
Apixaban intermediates,5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2- oxo-1-piperidinyl)phenyl]-2(1H)- pyridinone
Apixaban morpholine lactum impurity
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6- dihydropyridin-2(1H)-one
��,3-Morpholino-1-(4-(2-oxopiperidin-1- yl)phenyl)-5,6-dihydropyridin-2(1H)- oneethyl 7-chloro-8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylate | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C20H25N3O3 | [MDL Number]
MFCD19440881 | [MOL File]
545445-44-1.mol | [Molecular Weight]
355 |
| Chemical Properties | Back Directory | [Melting point ]
>199°C (dec.) | [Boiling point ]
625.0±55.0 °C(Predicted) | [density ]
1.267 | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
3.25±0.20(Predicted) | [color ]
Pale Beige | [InChI]
InChI=1S/C20H25N3O3/c24-19-5-1-2-10-22(19)16-6-8-17(9-7-16)23-11-3-4-18(20(23)25)21-12-14-26-15-13-21/h4,6-9H,1-3,5,10-15H2 | [InChIKey]
SCVWQFDPLBFZAP-UHFFFAOYSA-N | [SMILES]
C1(=O)N(C2=CC=C(N3CCCCC3=O)C=C2)CCC=C1N1CCOCC1 |
| Hazard Information | Back Directory | [Uses]
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropy can be used as an organic synthesis intermediate, mainly used in laboratory research and development and chemical production processes. | [Synthesis]
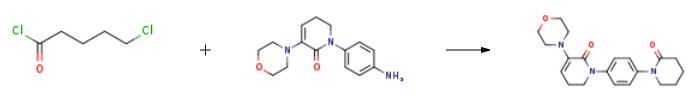
2 g compound C (compound Ib) and 50 ml acetonitrile were added into a three-necked flask to obtain a turbid solution. The turbid solution was stirred and cooled to 0?? C. in an ice bath. 1.75 g (6 eq) sodium hydroxide was added and stirred in the ice bath for 10 min. Then 1.9 ml of 5-chloro-valeryl chloride (2 eq) (diluted with 2 ml acetonitrile) was dropwise added while controlling the temperature to 0-5?? C. After completing the addition, the ice bath was removed and the temperature was naturally raised to 30?? C. The reaction was performed for 5 hours. After observing the absence of the starting material and the intermediate state of the reaction, the reaction solution was cooled to 0?? C. in an ice bath, and adjusted to a neutral pH with 6 N of hydrochloric acid. The reaction solution was concentrated to dryness, pulpifying for 1 hour after adding 8 ml saturated sodium bicarbonate solution, and filtered to obtain 2.29 g yellow solid. Yield: 87.5%. Purity: 95.3% (HPLC). EI-MS (m/z): 355.2. HNMR (400 MHz, DMSO, ppm) |?7.32 (d, J=8.8 Hz, 2H), 7.26 (d, J=8.8 Hz, 2H), 5.71 (t, J=4.8 Hz, 1H), 3.72-3.69 (m, 2H), 3.65-3.63 (m, 4H), 3.60-3.57 (m, 2H), 2.79-2.77 (m, 4H), 2.44-2.41 (m, 2H), 2.40-2.39 (m, 2H), 1.88-1.82 (m, 4H). |
|
|